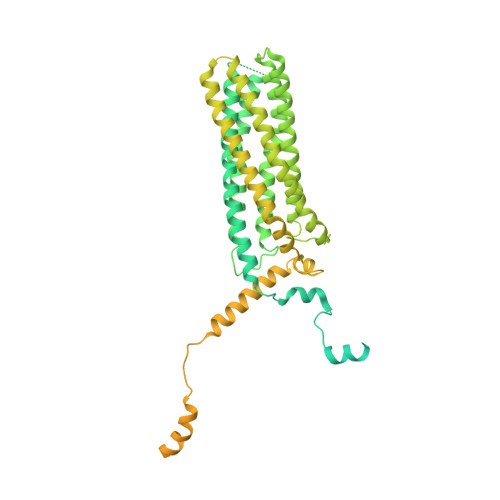
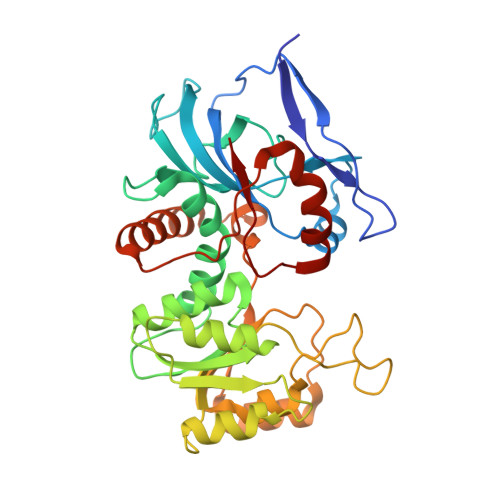
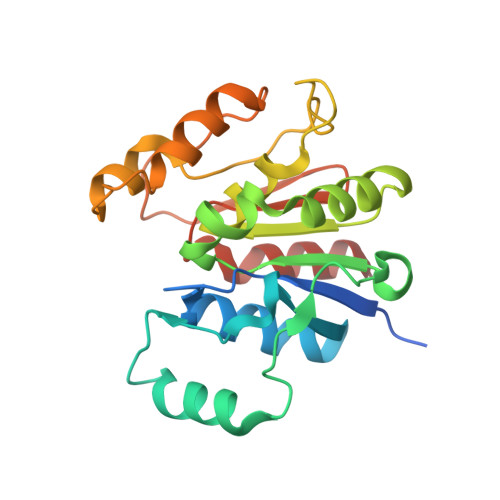
Structural basis of mRNA binding by the human FERRY Rab5 effector complex.
Quentin, D., Schuhmacher, J.S., Klink, B.U., Lauer, J., Shaikh, T.R., Huis In 't Veld, P.J., Welp, L.M., Urlaub, H., Zerial, M., Raunser, S.(2023) Mol Cell 83: 1856-1871.e9
- PubMed: 37267906
- DOI: https://doi.org/10.1016/j.molcel.2023.05.009
- Primary Citation of Related Structures:
7ND2, 8A3O, 8A3P - PubMed Abstract:
The pentameric FERRY Rab5 effector complex is a molecular link between mRNA and early endosomes in mRNA intracellular distribution. Here, we determine the cryo-EM structure of human FERRY. It reveals a unique clamp-like architecture that bears no resemblance to any known structure of Rab effectors. A combination of functional and mutational studies reveals that while the Fy-2 C-terminal coiled-coil acts as binding region for Fy-1/3 and Rab5, both coiled-coils and Fy-5 concur to bind mRNA. Mutations causing truncations of Fy-2 in patients with neurological disorders impair Rab5 binding or FERRY complex assembly. Thus, Fy-2 serves as a binding hub connecting all five complex subunits and mediating the binding to mRNA and early endosomes via Rab5. Our study provides mechanistic insights into long-distance mRNA transport and demonstrates that the particular architecture of FERRY is closely linked to a previously undescribed mode of RNA binding, involving coiled-coil domains.
- Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany.
Organizational Affiliation: