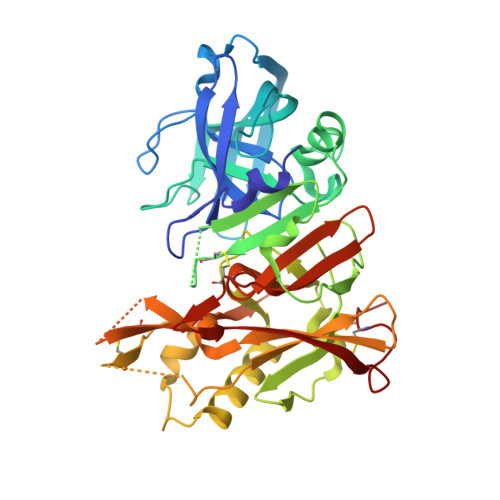
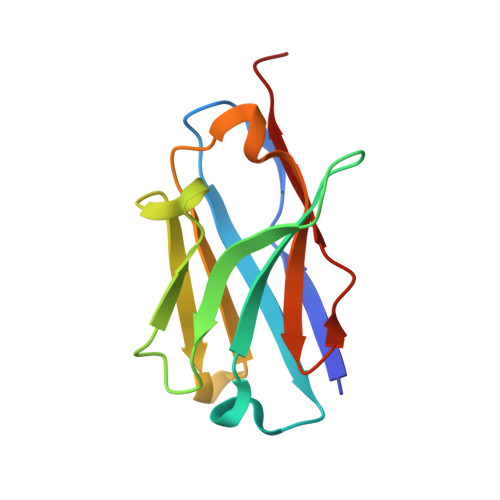
JNJ-67569762, A 2-Aminotetrahydropyridine-Based Selective BACE1 Inhibitor Targeting the S3 Pocket: From Discovery to Clinical Candidate.
Rombouts, F.J.R., Kusakabe, K.I., Alexander, R., Austin, N., Borghys, H., De Cleyn, M., Dhuyvetter, D., Gijsen, H.J.M., Hrupka, B., Jacobs, T., Jerhaoui, S., Lammens, L., Leclercq, L., Tsubone, K., Ueno, T., Morimoto, K., Einaru, S., Sumiyoshi, H., Van den Bergh, A., Vos, A., Surkyn, M., Teisman, A., Moechars, D.(2021) J Med Chem 64: 14175-14191
- PubMed: 34553934
- DOI: https://doi.org/10.1021/acs.jmedchem.1c00935
- Primary Citation of Related Structures:
7N4N, 7N66 - PubMed Abstract:
The discovery of a novel 2-aminotetrahydropyridine class of BACE1 inhibitors is described. Their pK a and lipophilicity were modulated by a pending sulfonyl group, while good permeability and brain penetration were achieved via intramolecular hydrogen bonding. BACE1 selectivity over BACE2 was achieved in the S3 pocket by a novel bicyclic ring system. An optimization addressing reactive metabolite formation, cardiovascular safety, and CNS toxicity is described, leading to the clinical candidate JNJ-67569762 ( 12 ), which gave robust dose-dependent BACE1-mediated amyloid β lowering without showing BACE2-dependent hair depigmentation in preclinical models. We show that 12 has a favorable projected human dose and PK and hence presented us with an opportunity to test a highly selective BACE1 inhibitor in humans. However, 12 was found to have a QT effect upon repeat dosing in dogs and its development was halted in favor of other selective leads, which will be reported in the future.
- Janssen Research & Development, Janssen Pharmaceutica N. V., Turnhoutseweg 30, Beerse B-2340, Belgium.
Organizational Affiliation: