Crystal Structure of ENOYL COA-HYDRATASE2 from Arabidopsis thaliana
Korasick, D.A., Powers, S.K., Jez, J.M., Strader, L.C.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Enoyl-CoA hydratase 2, peroxisomal | 329 | Arabidopsis thaliana | Mutation(s): 0 Gene Names: ECH2, At1g76150, T23E18.38, T23E18.9 EC: 4.2.1.119 | 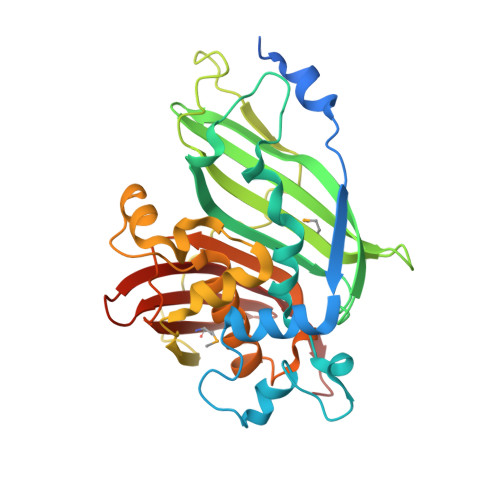 | |
UniProt | |||||
Find proteins for Q8VYI3 (Arabidopsis thaliana) Explore Q8VYI3 Go to UniProtKB: Q8VYI3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8VYI3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 134.744 | α = 90 |
| b = 134.744 | β = 90 |
| c = 127.849 | γ = 120 |
| Software Name | Purpose |
|---|---|
| SCALEPACK | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-3000 | data reduction |
| HKL-3000 | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R35 GM136338 |
| National Science Foundation (NSF, United States) | United States | IOS-1453750 |
| National Science Foundation (NSF, United States) | United States | MCB-1614539 |
| United States Department of Agriculture (USDA) | United States | MOW-2014-01877 |
| National Science Foundation (NSF, United States) | United States | 2011101911 |