Improved ligand discovery using micro-beam data collection at the edge of protein crystals
Soares, A.S., Jakoncic, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Endo-1,4-beta-xylanase | 190 | Trichoderma reesei | Mutation(s): 0 EC: 3.2.1.8 | 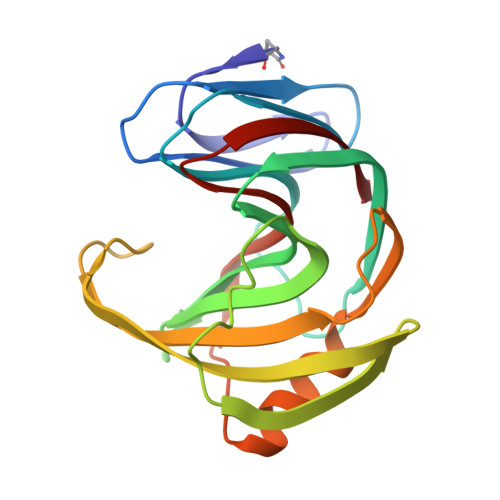 | |
UniProt | |||||
Find proteins for P36217 (Hypocrea jecorina (strain ATCC 56765 / BCRC 32924 / NRRL 11460 / Rut C-30)) Explore P36217 Go to UniProtKB: P36217 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P36217 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ARG (Subject of Investigation/LOI) Query on ARG | B [auth A] | ARGININE C6 H15 N4 O2 ODKSFYDXXFIFQN-BYPYZUCNSA-O |  | ||
| EDO Query on EDO | H [auth A], I [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| NA Query on NA | C [auth A], D [auth A], E [auth A], F [auth A], G [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| PCA Query on PCA | A | L-PEPTIDE LINKING | C5 H7 N O3 |  | GLN |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.41 | α = 90 |
| b = 38.99 | β = 107.64 |
| c = 57.11 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R21 GM129570 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | P30 GM133893 |
| Department of Energy (DOE, United States) | United States | KP 1605010 |
| Department of Energy (DOE, United States) | United States | DE SC0012704 |