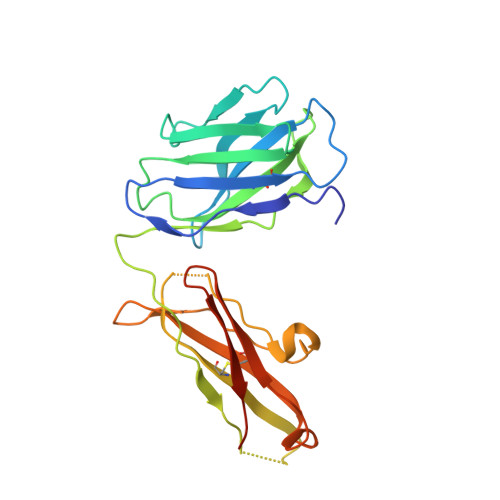
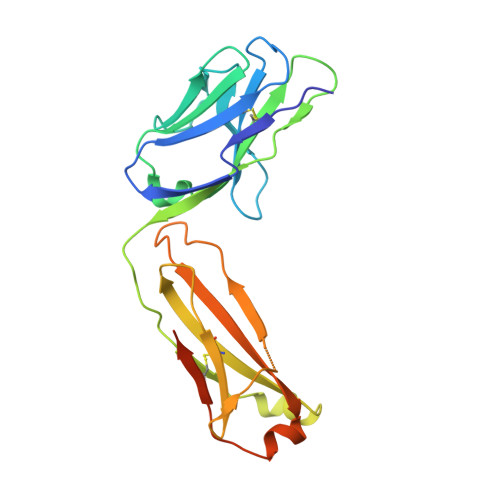
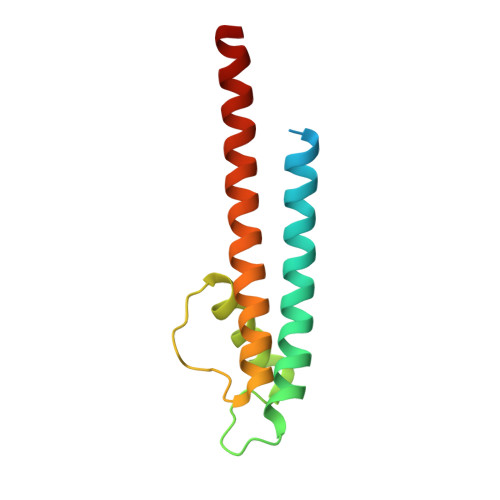
Engineering of a synthetic antibody fragment for structural and functional studies of K+ channels.
Rohaim, A., Slezak, T., Koh, Y.H., Blachowicz, L., Kossiakoff, A.A., Roux, B.(2022) J Gen Physiol 154
- PubMed: 35234830
- DOI: https://doi.org/10.1085/jgp.202112965
- Primary Citation of Related Structures:
7MDJ - PubMed Abstract:
Engineered antibody fragments (Fabs) have made major impacts on structural biology research, particularly to aid structural determination of membrane proteins. Nonetheless, Fabs generated by traditional monoclonal technology suffer from challenges of routine production and storage. Starting from the known IgG paratopes of an antibody that binds to the "turret loop" of the KcsA K+ channel, we engineered a synthetic Fab (sFab) based upon the highly stable Herceptin Fab scaffold, which can be recombinantly expressed in Escherichia coli and purified with single-step affinity chromatography. This synthetic Fab was used as a crystallization chaperone to obtain crystals of the KcsA channel that diffracted to a resolution comparable to that from the parent Fab. Furthermore, we show that the turret loop can be grafted into the unrelated voltage-gated Kv1.2-Kv2.1 channel and still strongly bind the engineered sFab, in support of the loop grafting strategy. Macroscopic electrophysiology recordings show that the sFab affects the activation and conductance of the chimeric voltage-gated channel. These results suggest that straightforward engineering of antibodies using recombinant formats can facilitate the rapid and scalable production of Fabs as structural biology tools and functional probes. The impact of this approach is expanded significantly based on the potential portability of the turret loop to a myriad of other K+ channels.
- Department of Biochemistry and Molecular Biology, University of Chicago, Chicago, IL.
Organizational Affiliation: