The internal aldimine form of the wild-type Salmonella Typhimurium Tryptophan Synthase in complex with cesium ion at the metal coordination site at 1.61 Angstrom resolution
Hilario, E., Dunn, M.F., Mueller, L.J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tryptophan synthase alpha chain | 268 | Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | Mutation(s): 0 Gene Names: trpA, STM1727 EC: 4.2.1.20 | 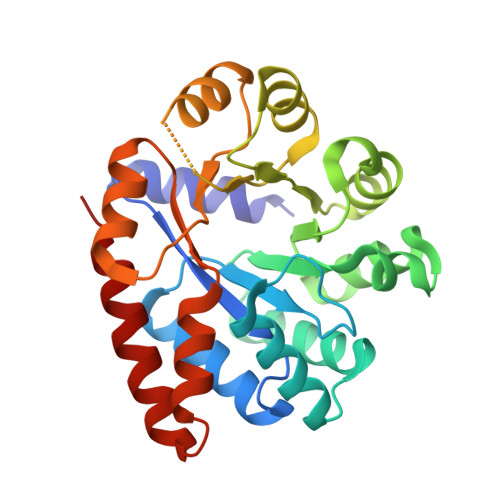 | |
UniProt | |||||
Find proteins for P00929 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore P00929 Go to UniProtKB: P00929 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00929 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tryptophan synthase beta chain | 397 | Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | Mutation(s): 0 Gene Names: trpB EC: 4.2.1.20 | 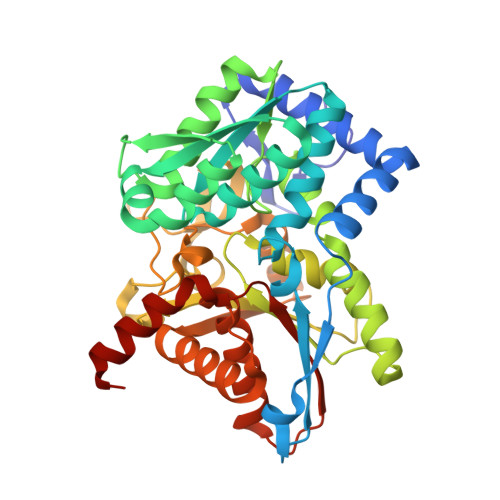 | |
UniProt | |||||
Find proteins for P0A2K1 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore P0A2K1 Go to UniProtKB: P0A2K1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A2K1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 6 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PLP (Subject of Investigation/LOI) Query on PLP | O [auth B] | PYRIDOXAL-5'-PHOSPHATE C8 H10 N O6 P NGVDGCNFYWLIFO-UHFFFAOYSA-N |  | ||
| CS (Subject of Investigation/LOI) Query on CS | IA [auth B], M [auth A], N [auth A], V [auth B], W [auth B] | CESIUM ION Cs NCMHKCKGHRPLCM-UHFFFAOYSA-N |  | ||
| PEG Query on PEG | H [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| DMS Query on DMS | AA [auth B] BA [auth B] CA [auth B] D [auth A] DA [auth B] | DIMETHYL SULFOXIDE C2 H6 O S IAZDPXIOMUYVGZ-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | C [auth A], HA [auth B], I [auth A], Y [auth B] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CL Query on CL | EA [auth B] FA [auth B] G [auth A] GA [auth B] L [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 182.332 | α = 90 |
| b = 58.379 | β = 94.77 |
| c = 67.229 | γ = 90 |
| Software Name | Purpose |
|---|---|
| xia2 | data reduction |
| SCALA | data scaling |
| MOLREP | phasing |
| DM | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Human Genome Research Institute (NIH/NHGRI) | United States | 2R01GM097569-06A1 |