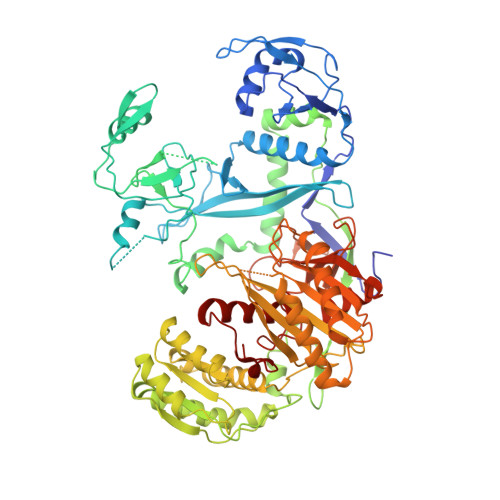
Structural basis for piRNA targeting.
Anzelon, T.A., Chowdhury, S., Hughes, S.M., Xiao, Y., Lander, G.C., MacRae, I.J.(2021) Nature 597: 285-289
- PubMed: 34471284
- DOI: https://doi.org/10.1038/s41586-021-03856-x
- Primary Citation of Related Structures:
7KX7, 7KX9 - PubMed Abstract:
PIWI proteins use PIWI-interacting RNAs (piRNAs) to identify and silence transposable elements and thereby maintain genome integrity between metazoan generations 1 . The targeting of transposable elements by PIWI has been compared to mRNA target recognition by Argonaute proteins 2,3 , which use microRNA (miRNA) guides, but the extent to which piRNAs resemble miRNAs is not known. Here we present cryo-electron microscopy structures of a PIWI-piRNA complex from the sponge Ephydatia fluviatilis with and without target RNAs, and a biochemical analysis of target recognition. Mirroring Argonaute, PIWI identifies targets using the piRNA seed region. However, PIWI creates a much weaker seed so that stable target association requires further piRNA-target pairing, making piRNAs less promiscuous than miRNAs. Beyond the seed, the structure of PIWI facilitates piRNA-target pairing in a manner that is tolerant of mismatches, leading to long-lived PIWI-piRNA-target interactions that may accumulate on transposable-element transcripts. PIWI ensures targeting fidelity by physically blocking the propagation of piRNA-target interactions in the absence of faithful seed pairing, and by requiring an extended piRNA-target duplex to reach an endonucleolytically active conformation. PIWI proteins thereby minimize off-targeting cellular mRNAs while defending against evolving genomic threats.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA, USA.
Organizational Affiliation: