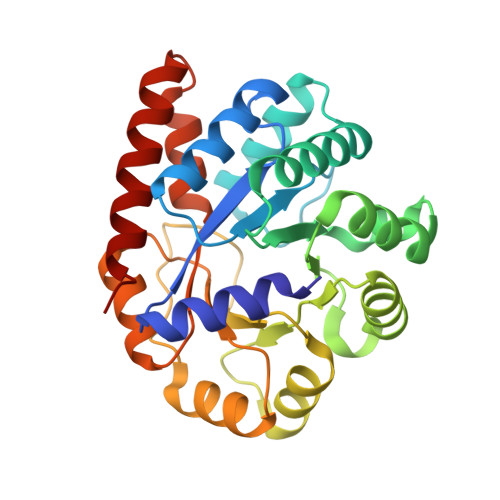
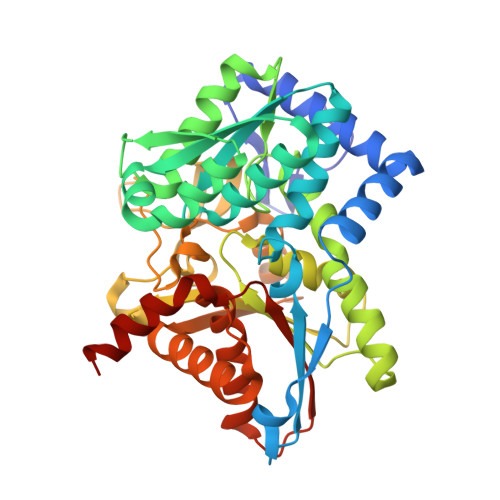
The internal aldimine form of the wild-type Tryptophan Synthase from Salmonella in complex with inhibitor N-(4'-trifluoromethoxybenzenesulfonyl)-2-amino-1-ethylphosphate (F9F) at the enzyme alpha-site and cesium ion at the metal coordination site at 1.47 Angstrom resolution.
Hilario, E., Dunn, M.F., Mueller, L.J.To be published.