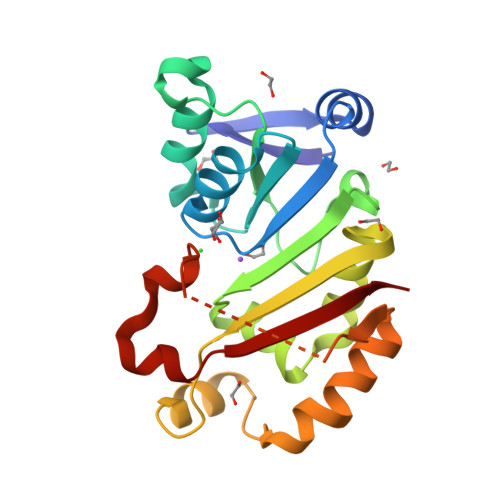
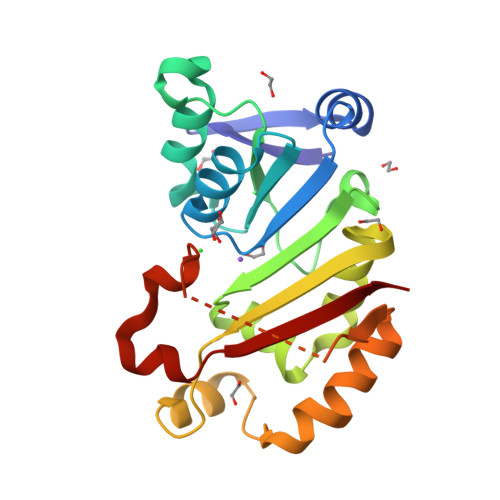
Functional and Structural Characterization of Acquired 16S rRNA Methyltransferase NpmB1 Conferring Pan-Aminoglycoside Resistance.
Kawai, A., Suzuki, M., Tsukamoto, K., Minato, Y., Doi, Y.(2021) Antimicrob Agents Chemother 65: e0100921-e0100921
- PubMed: 34310216
- DOI: https://doi.org/10.1128/AAC.01009-21
- Primary Citation of Related Structures:
7EHF - PubMed Abstract:
Posttranslational methylation of the A site of 16S rRNA at position A1408 leads to pan-aminoglycoside resistance encompassing both 4,5- and 4,6-disubstituted 2-deoxystreptamine (DOS) aminoglycosides. To date, NpmA is the only acquired enzyme with such a function. Here, we present the function and structure of NpmB1, whose sequence was identified in Escherichia coli genomes registered from the United Kingdom. NpmB1 possesses 40% amino acid identity with NpmA1 and confers resistance to all clinically relevant aminoglycosides, including 4,5-DOS agents. Phylogenetic analysis of NpmB1 and NpmB2, its single-amino-acid variant, revealed that the encoding gene was likely acquired by E. coli from a soil bacterium. The structure of NpmB1 suggests that it requires a structural change of the β6/7 linker in order to bind to 16S rRNA. These findings establish NpmB1 and NpmB2 as the second group of acquired pan-aminoglycoside resistance 16S rRNA methyltransferases.
Organizational Affiliation:
Department of Microbiology, Fujita Health Universitygrid.256115.4 School of Medicine, Aichi, Japan.