Structural insights into the potency and selectivity of covalent pan-FGFR inhibitors
Qu, L., Chen, X., Wei, H., Guo, M., Dai, S., Jiang, L., Li, J., Yue, S., Chen, Z., Chen, Y.(2022) Commun Chem 5: 5
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proto-oncogene tyrosine-protein kinase Src | A [auth B], B [auth A] | 286 | Gallus gallus | Mutation(s): 0 Gene Names: SRC EC: 2.7.10.2 | 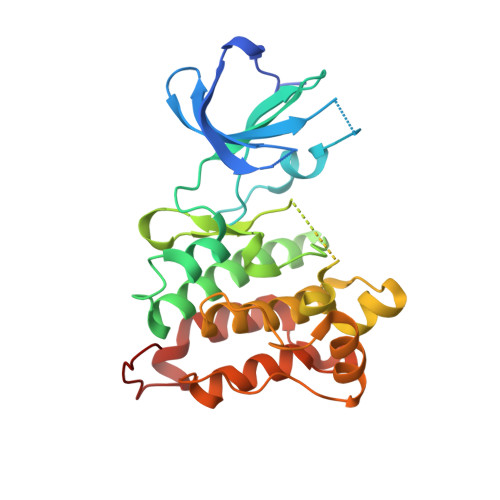 |
UniProt | |||||
Find proteins for P00523 (Gallus gallus) Explore P00523 Go to UniProtKB: P00523 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00523 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TZ0 (Subject of Investigation/LOI) Query on TZ0 | C [auth B], D [auth A] | 1-[(3S)-3-{4-amino-3-[(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl}pyrrolidin-1-yl]prop-2-en-1-one C22 H22 N6 O3 KEIPNCCJPRMIAX-HNNXBMFYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 41.969 | α = 100.64 |
| b = 63.184 | β = 90.61 |
| c = 73.137 | γ = 89.82 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 81570537 |
| National Natural Science Foundation of China (NSFC) | China | 81974074 |
| National Natural Science Foundation of China (NSFC) | China | 31900880 |