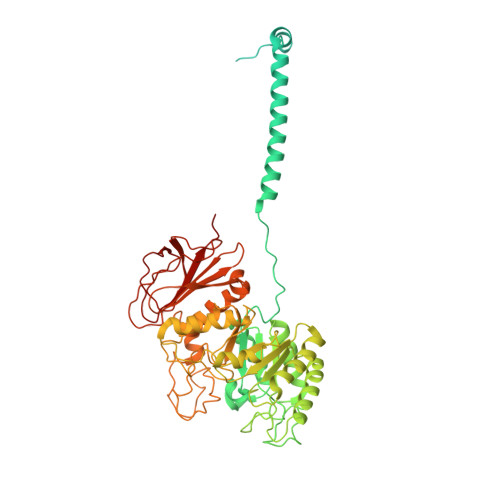
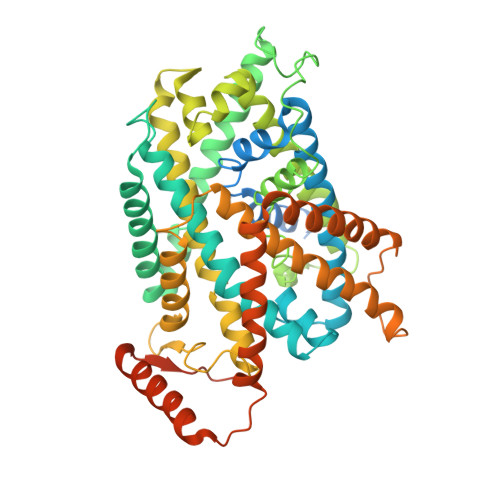
Structural insight into the substrate recognition and transport mechanism of the human LAT2-4F2hc complex.
Yan, R., Zhou, J., Li, Y., Lei, J., Zhou, Q.(2020) Cell Discov 6: 82-82
- PubMed: 33298890
- DOI: https://doi.org/10.1038/s41421-020-00207-4
- Primary Citation of Related Structures:
7CMH, 7CMI - Zhejiang Provincial Laboratory of Life Sciences and Biomedicine, Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, 18 Shilongshan Road, Hangzhou, Zhejiang 310024, China.
Organizational Affiliation: