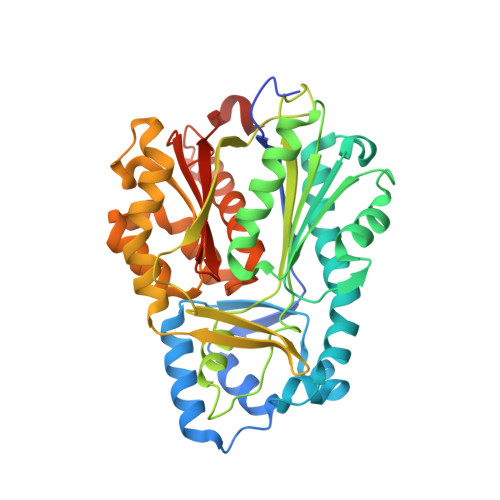
Crystal structure of benzophenone synthase from Garcinia mangostana L. pericarps reveals basis for substrate specificity and catalysis.
Songsiriritthigul, C., Nualkaew, N., Ketudat-Cairnsb, J., Chen, C.-J.(2020) Acta Crystallogr F Struct Biol Commun 76: 597-603