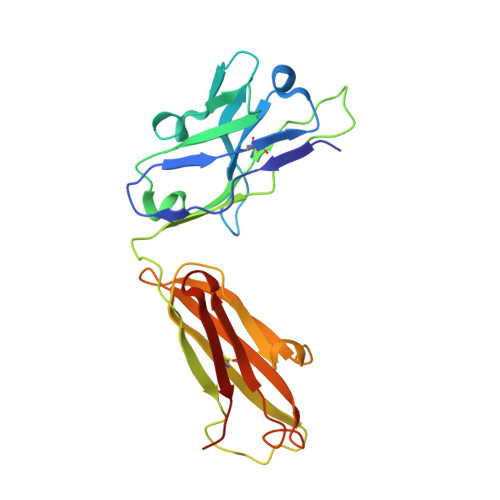
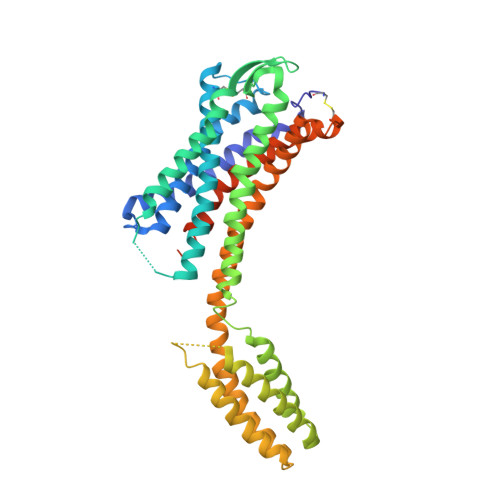
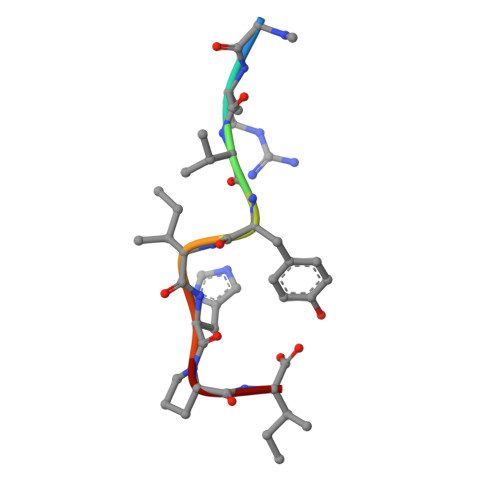
The discovery of a new antibody for BRIL-fused GPCR structure determination.
Miyagi, H., Asada, H., Suzuki, M., Takahashi, Y., Yasunaga, M., Suno, C., Iwata, S., Saito, J.I.(2020) Sci Rep 10: 11669-11669
- PubMed: 32669569
- DOI: https://doi.org/10.1038/s41598-020-68355-x
- Primary Citation of Related Structures:
7C61, 7C6A - PubMed Abstract:
G-protein-coupled receptors (GPCRs)-the largest family of cell-surface membrane proteins-mediate the intracellular signal transduction of many external ligands. Thus, GPCRs have become important drug targets. X-ray crystal structures of GPCRs are very useful for structure-based drug design (SBDD). Herein, we produced a new antibody (SRP2070) targeting the thermostabilised apocytochrome b562 from Escherichia coli M7W/H102I/R106L (BRIL). We found that a fragment of this antibody (SRP2070Fab) facilitated the crystallisation of the BRIL-tagged, ligand bound GPCRs, 5HT 1B and AT 2 R. Furthermore, the electron densities of the ligands were resolved, suggesting that SPR2070Fab is versatile and adaptable for GPCR SBDD. We anticipate that this new tool will significantly accelerate structure determination of other GPCRs and the design of small molecular drugs targeting them.
- R&D Division, Kyowa Kirin Co., Ltd., Tokyo, Japan.
Organizational Affiliation: