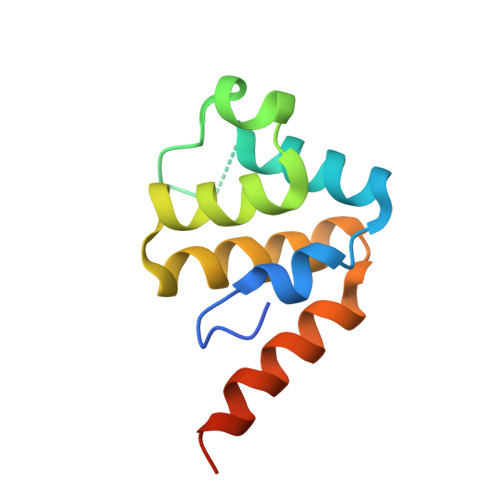
Crystal structure of the human NLRP9 pyrin domain reveals a bent N-terminal loop that may regulate inflammasome assembly.
Ha, H.J., Park, H.H.(2020) FEBS Lett 594: 2396-2405
- PubMed: 32542766
- DOI: https://doi.org/10.1002/1873-3468.13866
- Primary Citation of Related Structures:
7BSO - PubMed Abstract:
Members of the NLR family pyrin domain containing (NLRPs) are pattern recognition receptors that participate in innate immunity. They form inflammasomes, which are platforms for caspase-1 recruitment and activation. The NLRP pyrin domain (PYD) is critical for the assembly of inflammasomes due to its ability to mediate protein interactions. Despite intensive structural studies on inflammasomes with PYDs, the structure of the PYD of NLRP9-the least studied member of the family-remains unknown. Herein, we report the crystal structure of the human NLRP9 PYD at 2.1 Å resolution, which reveals a kinked N-terminal loop oriented toward the interior of the helical bundle. Based on our findings, we propose a regulatory role for the kinked N-terminal loop of NLRP9 PYD in inflammasome assembly.
- College of Pharmacy, Chung-Ang University, Seoul, Korea.
Organizational Affiliation: