CryoEM structure of the human sodium proton exchanger NHA2
Woehlert, D., Kuhlbrandt, W., Yildiz, O.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sodium/hydrogen exchanger 9B2 | 537 | Homo sapiens | Mutation(s): 0 Gene Names: SLC9B2, NHA2, NHEDC2 Membrane Entity: Yes | 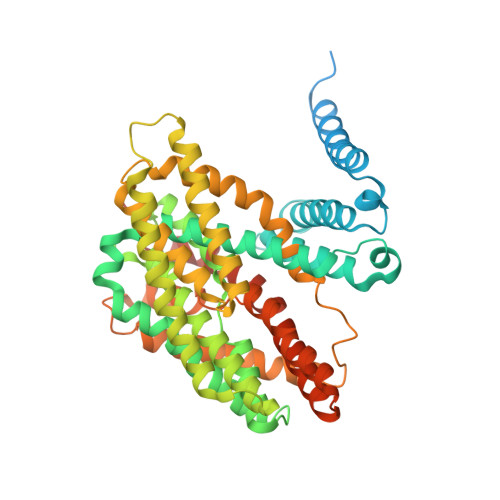 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q86UD5 (Homo sapiens) Explore Q86UD5 Go to UniProtKB: Q86UD5 | |||||
PHAROS: Q86UD5 GTEx: ENSG00000164038 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q86UD5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| POV Query on POV | D [auth A], M [auth A], N [auth B], P [auth B] | (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate C42 H82 N O8 P WTJKGGKOPKCXLL-PFDVCBLKSA-N |  | ||
| C14 Query on C14 | E [auth A] F [auth A] G [auth A] Q [auth B] R [auth B] | TETRADECANE C14 H30 BGHCVCJVXZWKCC-UHFFFAOYSA-N |  | ||
| UND Query on UND | H [auth A] I [auth A] J [auth A] K [auth A] L [auth A] | UNDECANE C11 H24 RSJKGSCJYJTIGS-UHFFFAOYSA-N |  | ||
| NA Query on NA | C [auth A], O [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | SFB807 |