14-3-3 sigma in complex with phosphopeptides
Soini, L., Leysen, S., Davis, J., Ottmann, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 14-3-3 protein sigma | 236 | Homo sapiens | Mutation(s): 0 Gene Names: SFN, HME1 | 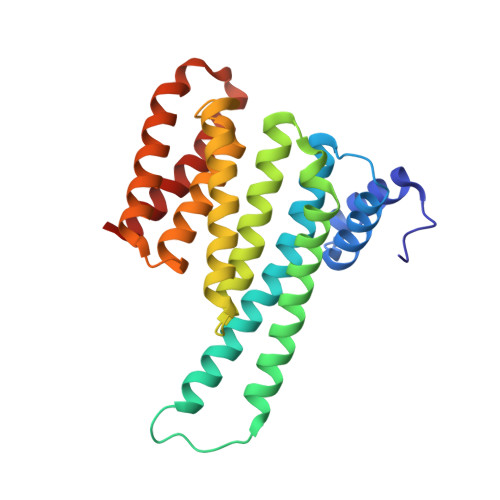 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P31947 (Homo sapiens) Explore P31947 Go to UniProtKB: P31947 | |||||
PHAROS: P31947 GTEx: ENSG00000175793 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P31947 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SHN3pT869 | B [auth P] | 9 | synthetic construct | Mutation(s): 0 | 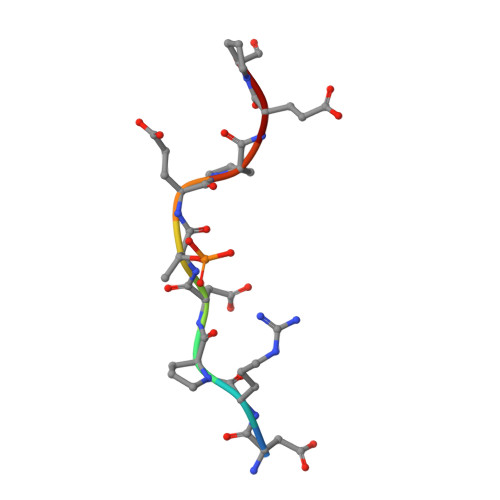 |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MG Query on MG | C [auth A], D [auth A], E [auth A], F [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TPO Query on TPO | B [auth P] | L-PEPTIDE LINKING | C4 H10 N O6 P |  | THR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 83.299 | α = 90 |
| b = 113.45 | β = 90 |
| c = 63.2 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Commission | Netherlands | 675179 |