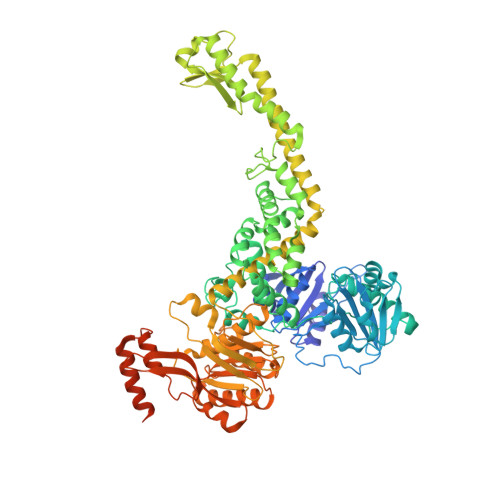
The selection process of licensing a DNA mismatch for repair.
Fernandez-Leiro, R., Bhairosing-Kok, D., Kunetsky, V., Laffeber, C., Winterwerp, H.H., Groothuizen, F., Fish, A., Lebbink, J.H.G., Friedhoff, P., Sixma, T.K., Lamers, M.H.(2021) Nat Struct Mol Biol 28: 373-381
- PubMed: 33820992
- DOI: https://doi.org/10.1038/s41594-021-00577-7
- Primary Citation of Related Structures:
7AI5, 7AI6, 7AI7, 7AIB, 7AIC - PubMed Abstract:
DNA mismatch repair detects and removes mismatches from DNA by a conserved mechanism, reducing the error rate of DNA replication by 100- to 1,000-fold. In this process, MutS homologs scan DNA, recognize mismatches and initiate repair. How the MutS homologs selectively license repair of a mismatch among millions of matched base pairs is not understood. Here we present four cryo-EM structures of Escherichia coli MutS that provide snapshots, from scanning homoduplex DNA to mismatch binding and MutL activation via an intermediate state. During scanning, the homoduplex DNA forms a steric block that prevents MutS from transitioning into the MutL-bound clamp state, which can only be overcome through kinking of the DNA at a mismatch. Structural asymmetry in all four structures indicates a division of labor between the two MutS monomers. Together, these structures reveal how a small conformational change from the homoduplex- to heteroduplex-bound MutS acts as a licensing step that triggers a dramatic conformational change that enables MutL binding and initiation of the repair cascade.
- MRC Laboratory of Medical Research, Cambridge, UK. rfleiro@cnio.es.
Organizational Affiliation: