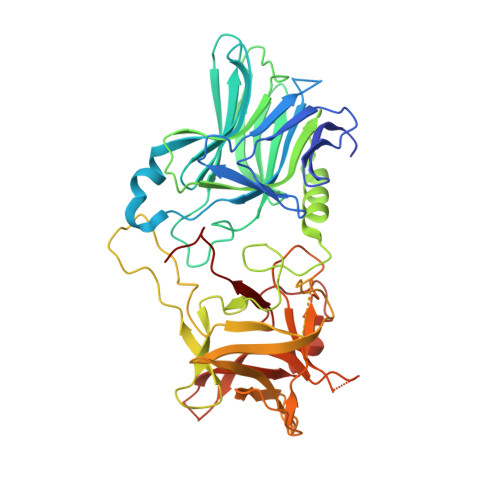
Structural and Biochemical Characterization of Botulinum Neurotoxin Subtype B2 Binding to Its Receptors.
Davies, J.R., Masuyer, G., Stenmark, P.(2020) Toxins (Basel) 12
- PubMed: 32957706
- DOI: https://doi.org/10.3390/toxins12090603
- Primary Citation of Related Structures:
6ZVM, 6ZVN - PubMed Abstract:
Botulinum neurotoxins (BoNTs) can be used therapeutically to treat a wide range of neuromuscular and neurological conditions. A collection of natural BoNT variants exists which can be classified into serologically distinct serotypes (BoNT/B), and further divided into subtypes (BoNT/B1, B2, …). BoNT subtypes share a high degree of sequence identity within the same serotype yet can display large variation in toxicity. One such example is BoNT/B2, which was isolated from Clostridium botulinum strain 111 in a clinical case of botulism, and presents a 10-fold lower toxicity than BoNT/B1. In an effort to understand the molecular mechanisms behind this difference in potency, we here present the crystal structures of BoNT/B2 in complex with the ganglioside receptor GD1a, and with the human synaptotagmin I protein receptor. We show, using receptor-binding assays, that BoNT/B2 has a slightly higher affinity for GD1a than BoNT/B1, and confirm its considerably weaker affinity for its protein receptors. Although the overall receptor-binding mechanism is conserved for both receptors, structural analysis suggests the lower affinity of BoNT/B2 is the result of key substitutions, where hydrophobic interactions important for synaptotagmin-binding are replaced by polar residues. This study provides a template to drive the development of future BoNT therapeutic molecules centered on assessing the natural subtype variations in receptor-binding that appears to be one of the principal stages driving toxicity.
- Department of Biochemistry and Biophysics, Stockholm University, SE-106 91 Stockholm, Sweden.
Organizational Affiliation: