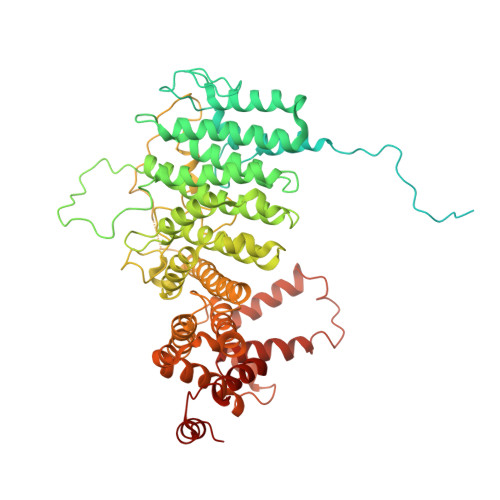
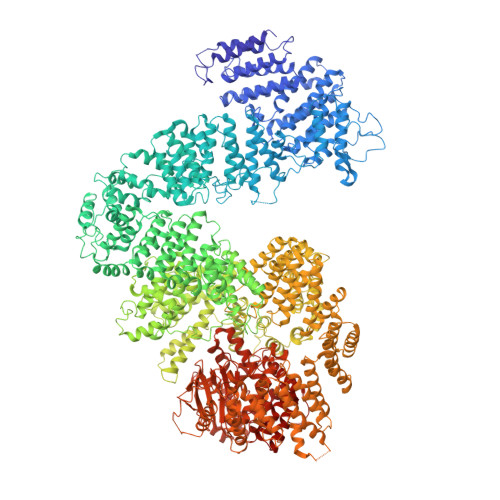
Mechanism of auto-inhibition and activation of Mec1 ATR checkpoint kinase.
Tannous, E.A., Yates, L.A., Zhang, X., Burgers, P.M.(2021) Nat Struct Mol Biol 28: 50-61
- PubMed: 33169019
- DOI: https://doi.org/10.1038/s41594-020-00522-0
- Primary Citation of Related Structures:
6Z2W, 6Z2X, 6Z3A - PubMed Abstract:
In response to DNA damage or replication fork stalling, the basal activity of Mec1 ATR is stimulated in a cell-cycle-dependent manner, leading to cell-cycle arrest and the promotion of DNA repair. Mec1 ATR dysfunction leads to cell death in yeast and causes chromosome instability and embryonic lethality in mammals. Thus, ATR is a major target for cancer therapies in homologous recombination-deficient cancers. Here we identify a single mutation in Mec1, conserved in ATR, that results in constitutive activity. Using cryo-electron microscopy, we determine the structures of this constitutively active form (Mec1(F2244L)-Ddc2) at 2.8 Å and the wild type at 3.8 Å, both in complex with Mg 2+ -AMP-PNP. These structures yield a near-complete atomic model for Mec1-Ddc2 and uncover the molecular basis for low basal activity and the conformational changes required for activation. Combined with biochemical and genetic data, we discover key regulatory regions and propose a Mec1 activation mechanism.
- Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, Saint Louis, MO, USA.
Organizational Affiliation: