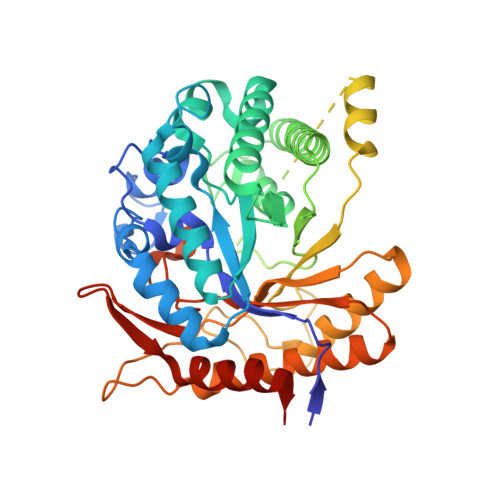
Heme-binding enables allosteric modulation in an ancient TIM-barrel glycosidase.
Gamiz-Arco, G., Gutierrez-Rus, L.I., Risso, V.A., Ibarra-Molero, B., Hoshino, Y., Petrovic, D., Justicia, J., Cuerva, J.M., Romero-Rivera, A., Seelig, B., Gavira, J.A., Kamerlin, S.C.L., Gaucher, E.A., Sanchez-Ruiz, J.M.(2021) Nat Commun 12: 380-380
- PubMed: 33452262
- DOI: https://doi.org/10.1038/s41467-020-20630-1
- Primary Citation of Related Structures:
6Z1H, 6Z1M - PubMed Abstract:
Glycosidases are phylogenetically widely distributed enzymes that are crucial for the cleavage of glycosidic bonds. Here, we present the exceptional properties of a putative ancestor of bacterial and eukaryotic family-1 glycosidases. The ancestral protein shares the TIM-barrel fold with its modern descendants but displays large regions with greatly enhanced conformational flexibility. Yet, the barrel core remains comparatively rigid and the ancestral glycosidase activity is stable, with an optimum temperature within the experimental range for thermophilic family-1 glycosidases. None of the ∼5500 reported crystallographic structures of ∼1400 modern glycosidases show a bound porphyrin. Remarkably, the ancestral glycosidase binds heme tightly and stoichiometrically at a well-defined buried site. Heme binding rigidifies this TIM-barrel and allosterically enhances catalysis. Our work demonstrates the capability of ancestral protein reconstructions to reveal valuable but unexpected biomolecular features when sampling distant sequence space. The potential of the ancestral glycosidase as a scaffold for custom catalysis and biosensor engineering is discussed.
- Departamento de Quimica Fisica. Facultad de Ciencias, Unidad de Excelencia de Quimica Aplicada a Biomedicina y Medioambiente (UEQ), Universidad de Granada, 18071, Granada, Spain.
Organizational Affiliation: