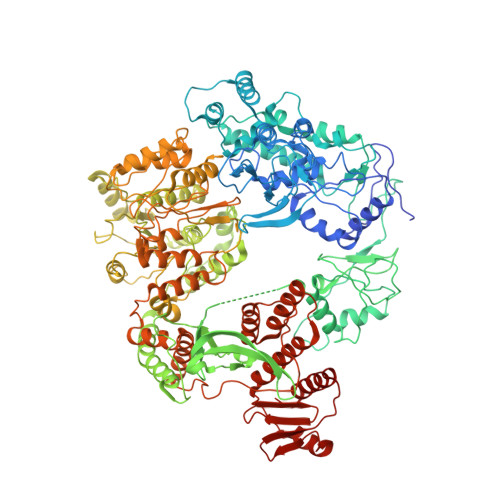
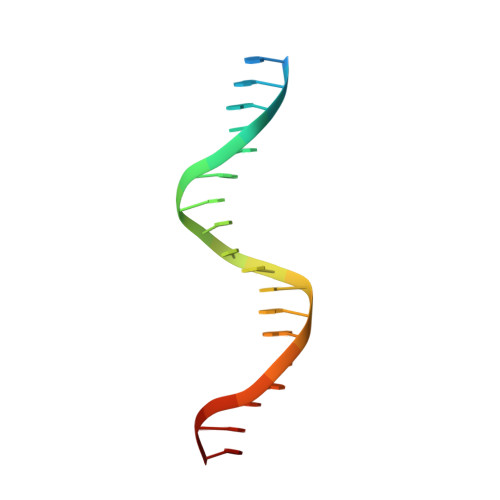
Molecular determinants for dsDNA translocation by the transcription-repair coupling and evolvability factor Mfd.
Brugger, C., Zhang, C., Suhanovsky, M.M., Kim, D.D., Sinclair, A.N., Lyumkis, D., Deaconescu, A.M.(2020) Nat Commun 11: 3740-3740
- PubMed: 32719356
- DOI: https://doi.org/10.1038/s41467-020-17457-1
- Primary Citation of Related Structures:
6XEO - PubMed Abstract:
Mfd couples transcription to nucleotide excision repair, and acts on RNA polymerases when elongation is impeded. Depending on impediment severity, this action results in either transcription termination or elongation rescue, which rely on ATP-dependent Mfd translocation on DNA. Due to its role in antibiotic resistance, Mfd is also emerging as a prime target for developing anti-evolution drugs. Here we report the structure of DNA-bound Mfd, which reveals large DNA-induced structural changes that are linked to the active site via ATPase motif VI. These changes relieve autoinhibitory contacts between the N- and C-termini and unmask UvrA recognition determinants. We also demonstrate that translocation relies on a threonine in motif Ic, widely conserved in translocases, and a family-specific histidine near motif IVa, reminiscent of the "arginine clamp" of RNA helicases. Thus, Mfd employs a mode of DNA recognition that at its core is common to ss/ds translocases that act on DNA or RNA.
- Department of Molecular Biology, Cell Biology and Biochemistry, Brown University, Providence, RI, 02903, USA.
Organizational Affiliation: