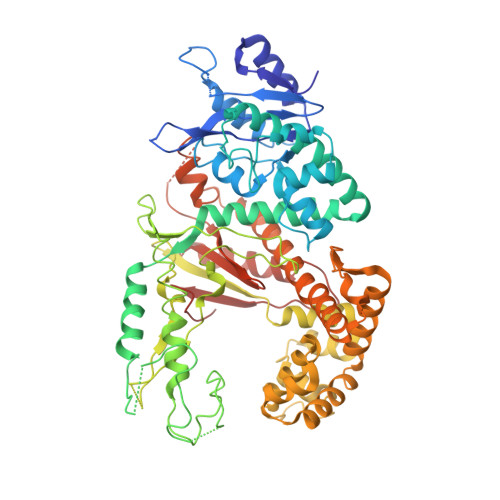
Pol theta reverse transcribes RNA and promotes RNA-templated DNA repair.
Chandramouly, G., Zhao, J., McDevitt, S., Rusanov, T., Hoang, T., Borisonnik, N., Treddinick, T., Lopezcolorado, F.W., Kent, T., Siddique, L.A., Mallon, J., Huhn, J., Shoda, Z., Kashkina, E., Brambati, A., Stark, J.M., Chen, X.S., Pomerantz, R.T.(2021) Sci Adv 7
- PubMed: 34117057
- DOI: https://doi.org/10.1126/sciadv.abf1771
- Primary Citation of Related Structures:
6XBU - PubMed Abstract:
Genome-embedded ribonucleotides arrest replicative DNA polymerases (Pols) and cause DNA breaks. Whether mammalian DNA repair Pols efficiently use template ribonucleotides and promote RNA-templated DNA repair synthesis remains unknown. We find that human Polθ reverse transcribes RNA, similar to retroviral reverse transcriptases (RTs). Polθ exhibits a significantly higher velocity and fidelity of deoxyribonucleotide incorporation on RNA versus DNA. The 3.2-Å crystal structure of Polθ on a DNA/RNA primer-template with bound deoxyribonucleotide reveals that the enzyme undergoes a major structural transformation within the thumb subdomain to accommodate A-form DNA/RNA and forms multiple hydrogen bonds with template ribose 2'-hydroxyl groups like retroviral RTs. Last, we find that Polθ promotes RNA-templated DNA repair in mammalian cells. These findings suggest that Polθ was selected to accommodate template ribonucleotides during DNA repair.
- Department of Biochemistry and Molecular Biology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA.
Organizational Affiliation: