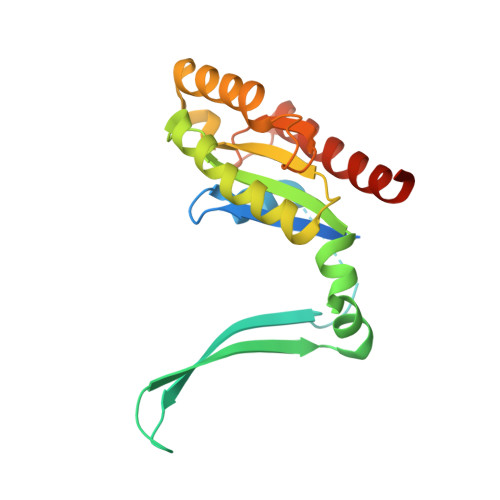
Structural basis for conformational plasticity in the GTPase domain of the Parkinson's disease-associated protein LRRK2
Wu, C.X., Liao, J., Park, Y., Hoang, N.C., Engel, V.A., Sanishvili, R., Takagi, Y., Johnson, S.M., Wang, M., Federici, M., Nichols, R.J., Cookson, M.R., Hoang, Q.Q.To be published.