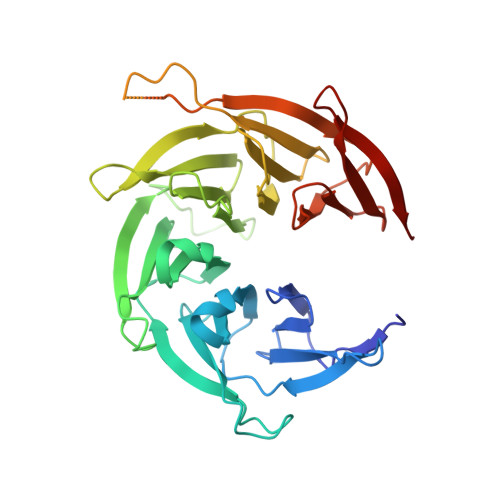
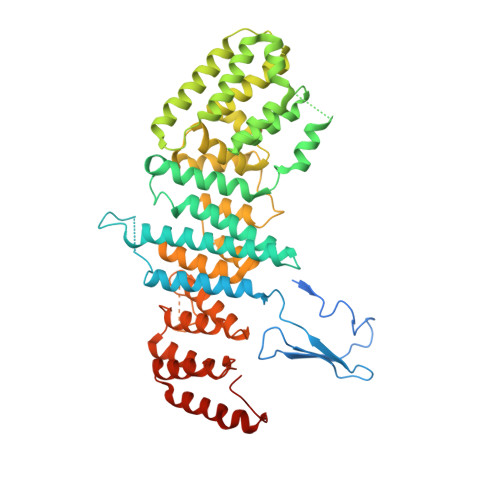
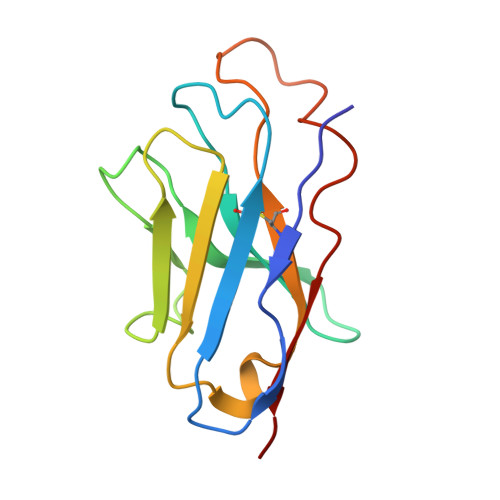
A nanobody suite for yeast scaffold nucleoporins provides details of the nuclear pore complex structure.
Nordeen, S.A., Andersen, K.R., Knockenhauer, K.E., Ingram, J.R., Ploegh, H.L., Schwartz, T.U.(2020) Nat Commun 11: 6179-6179
- PubMed: 33268786
- DOI: https://doi.org/10.1038/s41467-020-19884-6
- Primary Citation of Related Structures:
6X06, 6X07, 6X08 - PubMed Abstract:
Nuclear pore complexes (NPCs) are the main conduits for molecular exchange across the nuclear envelope. The NPC is a modular assembly of ~500 individual proteins, called nucleoporins or nups. Most scaffolding nups are organized in two multimeric subcomplexes, the Nup84 or Y complex and the Nic96 or inner ring complex. Working in S. cerevisiae, and to study the assembly of these two essential subcomplexes, we here develop a set of twelve nanobodies that recognize seven constituent nucleoporins of the Y and Nic96 complexes. These nanobodies all bind specifically and with high affinity. We present structures of several nup-nanobody complexes, revealing their binding sites. Additionally, constitutive expression of the nanobody suite in S. cerevisiae detect accessible and obstructed surfaces of the Y complex and Nic96 within the NPC. Overall, this suite of nanobodies provides a unique and versatile toolkit for the study of the NPC.
- Department of Biology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Organizational Affiliation: