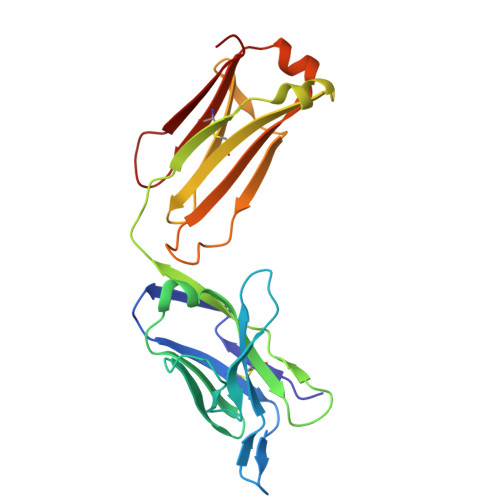
Antibody-Based Inhibition of Pathogenic New World Hemorrhagic Fever Mammarenaviruses by Steric Occlusion of the Human Transferrin Receptor 1 Apical Domain.
Ferrero, S., Flores, M.D., Short, C., Vazquez, C.A., Clark, L.E., Ziegenbein, J., Zink, S., Fuentes, D., Payes, C., Batto, M.V., Collazo, M., Garcia, C.C., Abraham, J., Cordo, S.M., Rodriguez, J.A., Helguera, G.(2021) J Virol 95: e0186820-e0186820
- PubMed: 34132574
- DOI: https://doi.org/10.1128/JVI.01868-20
- Primary Citation of Related Structures:
6WX1 - PubMed Abstract:
Pathogenic clade B New World mammarenaviruses (NWM) can cause Argentine, Venezuelan, Brazilian, and Bolivian hemorrhagic fevers. Sequence variability among NWM glycoproteins (GP) poses a challenge to the development of broadly neutralizing therapeutics against the entire clade of viruses. However, blockade of their shared binding site on the apical domain of human transferrin receptor 1 (hTfR1/CD71) presents an opportunity for the development of effective and broadly neutralizing therapeutics. Here, we demonstrate that the murine monoclonal antibody OKT9, which targets the apical domain of hTfR1, can sterically block cellular entry by viral particles presenting clade B NWM glycoproteins (GP1-GP2). OKT9 blockade is also effective against viral particles pseudotyped with glycoproteins of a recently identified pathogenic Sabia-like virus. With nanomolar affinity for hTfR1, the OKT9 antigen binding fragment (OKT9-Fab) sterically blocks clade B NWM-GP1s and reduces infectivity of an attenuated strain of Junin virus. Binding of OKT9 to the hTfR1 ectodomain in its soluble, dimeric state produces stable assemblies that are observable by negative-stain electron microscopy. A model of the OKT9-sTfR1 complex, informed by the known crystallographic structure of sTfR1 and a newly determined structure of the OKT9 antigen binding fragment (Fab), suggests that OKT9 and the Machupo virus GP1 share a binding site on the hTfR1 apical domain. The structural basis for this interaction presents a framework for the design and development of high-affinity, broadly acting agents targeting clade B NWMs. IMPORTANCE Pathogenic clade B NWMs cause grave infectious diseases, the South American hemorrhagic fevers. Their etiological agents are Junin (JUNV), Guanarito (GTOV), Sabiá (SABV), Machupo (MACV), Chapare (CHAV), and a new Sabiá-like (SABV-L) virus recently identified in Brazil. These are priority A pathogens due to their high infectivity and mortality, their potential for person-to-person transmission, and the limited availability of effective therapeutics and vaccines to curb their effects. While low homology between surface glycoproteins of NWMs foils efforts to develop broadly neutralizing therapies targeting NWMs, this work provides structural evidence that OKT9, a monoclonal antibody targeting a single NWM glycoprotein binding site on hTfR1, can efficiently prevent their entry into cells.
- Laboratory of Pharmaceutical Biotechnology, Instituto de Biología y Medicina Experimental (IBYME-CONICET), Buenos Aires, Argentina.
Organizational Affiliation: