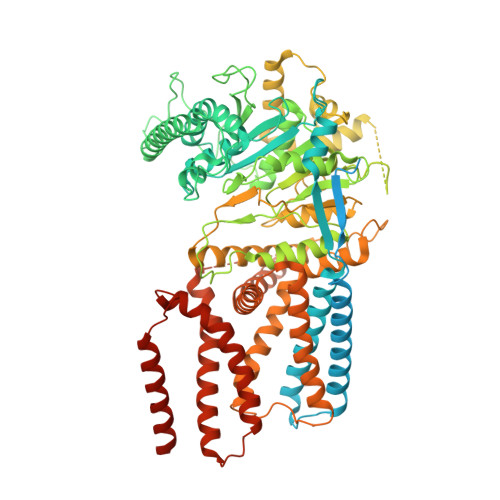
Architecture of a catalytically active homotrimeric plant cellulose synthase complex.
Purushotham, P., Ho, R., Zimmer, J.(2020) Science 369: 1089-1094
- PubMed: 32646917
- DOI: https://doi.org/10.1126/science.abb2978
- Primary Citation of Related Structures:
6WLB - PubMed Abstract:
Cellulose is an essential plant cell wall component and represents the most abundant biopolymer on Earth. Supramolecular plant cellulose synthase complexes organize multiple linear glucose polymers into microfibrils as load-bearing wall components. We determined the structure of a poplar cellulose synthase CesA homotrimer that suggests a molecular basis for cellulose microfibril formation. This complex, stabilized by cytosolic plant-conserved regions and helical exchange within the transmembrane segments, forms three channels occupied by nascent cellulose polymers. Secretion steers the polymers toward a common exit point, which could facilitate protofibril formation. CesA's N-terminal domains assemble into a cytosolic stalk that interacts with a microtubule-tethering protein and may thus be involved in CesA localization. Our data suggest how cellulose synthase complexes assemble and provide the molecular basis for plant cell wall engineering.
- Department of Molecular Physiology and Biological Physics, University of Virginia School of Medicine, 480 Ray C. Hunt Dr., Charlottesville, VA 22903, USA.
Organizational Affiliation: