Crystal structures of HyBcl-2-4 with HyBak1 BH3 and HyBax BH3
Banjara, S., Sa, J.D., Hinds, M.G., Kvansakul, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Maltodextrin-binding protein | 371 | Escherichia coli | Mutation(s): 0 Gene Names: DAH37_23060 | 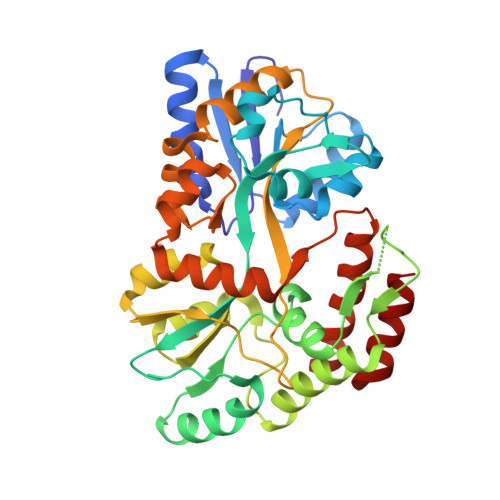 | |
UniProt | |||||
Find proteins for P0AEX9 (Escherichia coli (strain K12)) Explore P0AEX9 Go to UniProtKB: P0AEX9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0AEX9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bcl-2-like 4 | 162 | Hydra vulgaris | Mutation(s): 0 Gene Names: BCL2L1 | 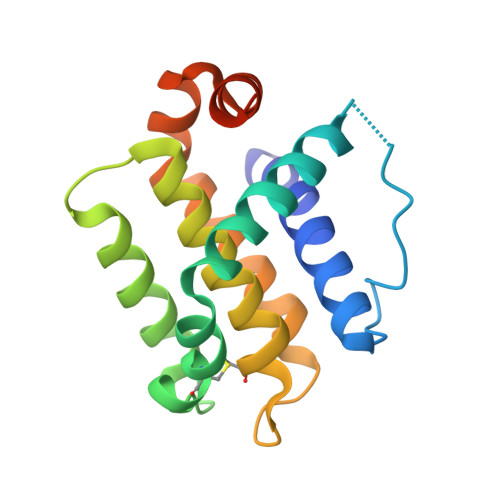 | |
UniProt | |||||
Find proteins for A7LM80 (Hydra vulgaris) Explore A7LM80 Go to UniProtKB: A7LM80 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A7LM80 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bak | 26 | Hydra vulgaris | Mutation(s): 0 | 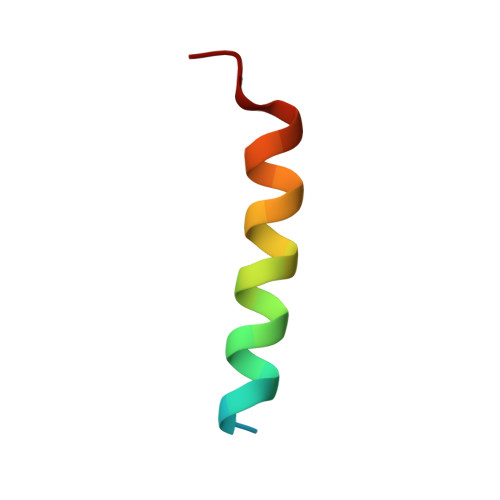 | |
UniProt | |||||
Find proteins for A1E3K5 (Hydra vulgaris) Explore A1E3K5 Go to UniProtKB: A1E3K5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A1E3K5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ACT Query on ACT | E [auth B], F [auth B] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900009 Query on PRD_900009 | D | alpha-maltotriose | Oligosaccharide / Nutrient |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 54.245 | α = 90 |
| b = 73.65 | β = 90 |
| c = 140.8 | γ = 90 |
| Software Name | Purpose |
|---|---|
| xia2 | data processing |
| PHASER | phasing |
| DIALS | data reduction |
| Aimless | data scaling |
| Coot | model building |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Australian Research Council (ARC) | Australia | DP190103591 |