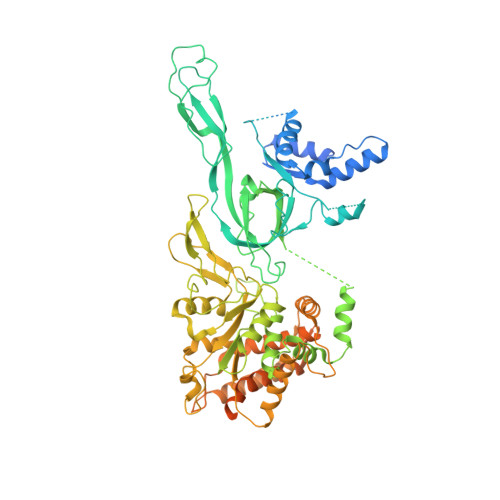
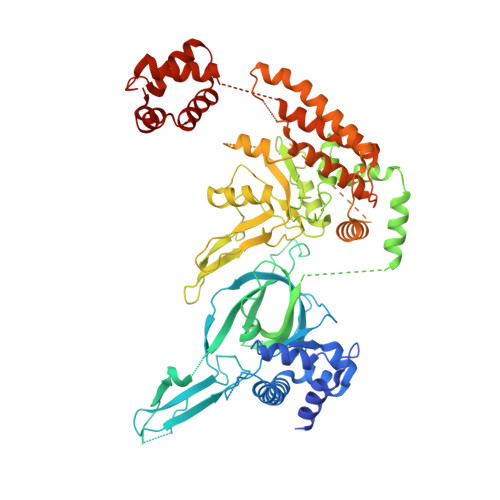
Structural mechanism of helicase loading onto replication origin DNA by ORC-Cdc6.
Yuan, Z., Schneider, S., Dodd, T., Riera, A., Bai, L., Yan, C., Magdalou, I., Ivanov, I., Stillman, B., Li, H., Speck, C.(2020) Proc Natl Acad Sci U S A 117: 17747-17756
- PubMed: 32669428
- DOI: https://doi.org/10.1073/pnas.2006231117
- Primary Citation of Related Structures:
6WGC, 6WGF, 6WGG, 6WGI - PubMed Abstract:
DNA replication origins serve as sites of replicative helicase loading. In all eukaryotes, the six-subunit origin recognition complex (Orc1-6; ORC) recognizes the replication origin. During late M-phase of the cell-cycle, Cdc6 binds to ORC and the ORC-Cdc6 complex loads in a multistep reaction and, with the help of Cdt1, the core Mcm2-7 helicase onto DNA. A key intermediate is the ORC-Cdc6-Cdt1-Mcm2-7 (OCCM) complex in which DNA has been already inserted into the central channel of Mcm2-7. Until now, it has been unclear how the origin DNA is guided by ORC-Cdc6 and inserted into the Mcm2-7 hexamer. Here, we truncated the C-terminal winged-helix-domain (WHD) of Mcm6 to slow down the loading reaction, thereby capturing two loading intermediates prior to DNA insertion in budding yeast. In "semi-attached OCCM," the Mcm3 and Mcm7 WHDs latch onto ORC-Cdc6 while the main body of the Mcm2-7 hexamer is not connected. In "pre-insertion OCCM," the main body of Mcm2-7 docks onto ORC-Cdc6, and the origin DNA is bent and positioned adjacent to the open DNA entry gate, poised for insertion, at the Mcm2-Mcm5 interface. We used molecular simulations to reveal the dynamic transition from preloading conformers to the loaded conformers in which the loading of Mcm2-7 on DNA is complete and the DNA entry gate is fully closed. Our work provides multiple molecular insights into a key event of eukaryotic DNA replication.
- Structural Biology Program, Van Andel Institute, Grand Rapids, MI 49503.
Organizational Affiliation: