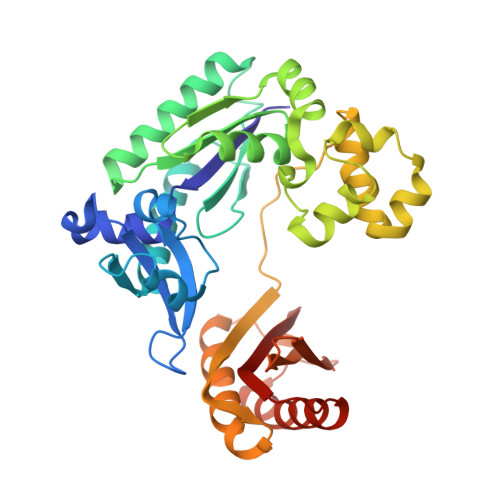
Promutagenic bypass of 7,8-dihydro-8-oxoadenine by translesion synthesis DNA polymerase Dpo4.
Jung, H., Lee, S.(2020) Biochem J 477: 2859-2871
- PubMed: 32686822
- DOI: https://doi.org/10.1042/BCJ20200449
- Primary Citation of Related Structures:
6VG6, 6VGM - PubMed Abstract:
Reactive oxygen species induced by ionizing radiation and metabolic pathways generate 7,8-dihydro-8-oxoguanine (oxoG) and 7,8-dihydro-8-oxoadenine (oxoA) as two major forms of oxidative damage. The mutagenicity of oxoG, which promotes G to T transversions, is attributed to the lesion's conformational flexibility that enables Hoogsteen base pairing with dATP in the confines of DNA polymerases. The mutagenesis mechanism of oxoA, which preferentially causes A to C transversions, remains poorly characterized. While structures for oxoA bypass by human DNA polymerases are available, that of prokaryotic DNA polymerases have not been reported. Herein, we report kinetic and structural characterizations of Sulfolobus solfataricus Dpo4 incorporating a nucleotide opposite oxoA. Our kinetic studies show oxoA at the templating position reduces the replication fidelity by ∼560-fold. The catalytic efficiency of the oxoA:dGTP insertion is ∼300-fold greater than that of the dA:dGTP insertion, highlighting the promutagenic nature of oxoA. The relative efficiency of the oxoA:dGTP misincorporation is ∼5-fold greater than that of the oxoG:dATP misincorporation, suggesting the mutagenicity of oxoA is comparable to that of oxoG. In the Dpo4 replicating base pair site, oxoA in the anti-conformation forms a Watson-Crick base pair with an incoming dTTP, while oxoA in the syn-conformation assumes Hoogsteen base pairing with an incoming dGTP, displaying the dual coding potential of the lesion. Within the Dpo4 active site, the oxoA:dGTP base pair adopts a Watson-Crick-like geometry, indicating Dpo4 influences the oxoA:dGTP base pair conformation. Overall, the results reported here provide insights into the miscoding properties of the major oxidative adenine lesion during translesion synthesis.
- The Division of Chemical Biology and Medicinal Chemistry, College of Pharmacy, The University of Texas at Austin, Austin, Texas 78712, U.S.A.
Organizational Affiliation: