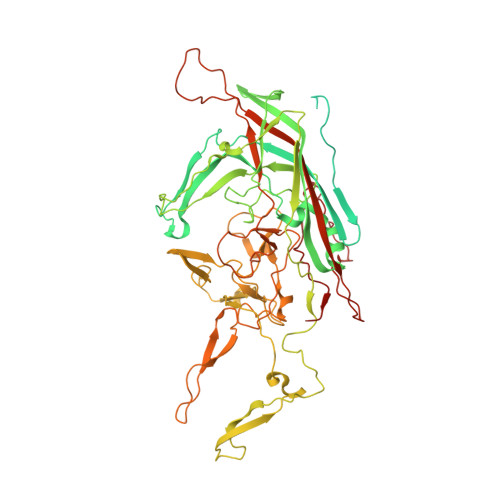
Structure of the AAVhu.37 capsid by cryoelectron microscopy.
Kaelber, J.T., Yost, S.A., Webber, K.A., Firlar, E., Liu, Y., Danos, O., Mercer, A.C.(2020) Acta Crystallogr F Struct Biol Commun 76: 58-64
- PubMed: 32039886
- DOI: https://doi.org/10.1107/S2053230X20000308
- Primary Citation of Related Structures:
6U95 - PubMed Abstract:
Adeno-associated viruses (AAVs) are used as in vivo gene-delivery vectors in gene-therapy products and have been heavily investigated for numerous indications. Over 100 naturally occurring AAV serotypes and variants have been isolated from primate samples. Many reports have described unique properties of these variants (for instance, differences in potency, target cell or evasion of the immune response), despite high amino-acid sequence conservation. AAVhu.37 is of interest for clinical applications owing to its proficient transduction of the liver and central nervous system. The sequence identity of the AAVhu.37 VP1 to the well characterized AAVrh.10 serotype, for which no structure is available, is greater than 98%. Here, the structure of the AAVhu.37 capsid at 2.56 Å resolution obtained via single-particle cryo-electron microscopy is presented.
- Institute of Quantitative Biomedicine and Rutgers New Jersey CryoEM/CryoET Core Facility, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA.
Organizational Affiliation: