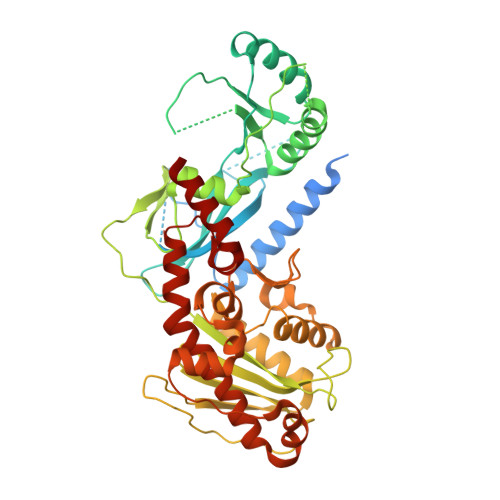
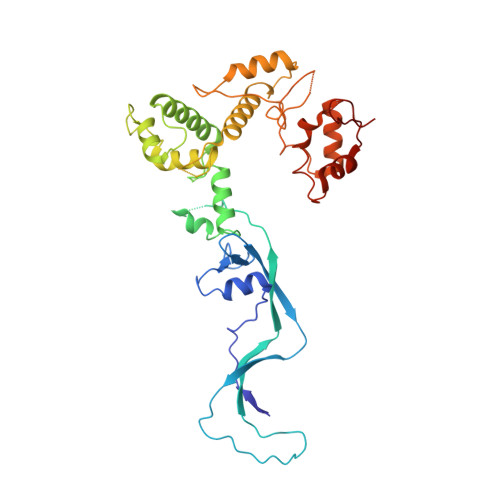
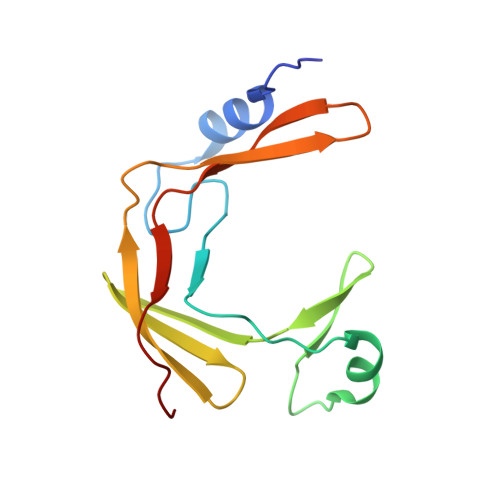
Ctf18-RFC and DNA Pol ε form a stable leading strand polymerase/clamp loader complex required for normal and perturbed DNA replication.
Stokes, K., Winczura, A., Song, B., Piccoli, G., Grabarczyk, D.B.(2020) Nucleic Acids Res 48: 8128-8145
- PubMed: 32585006
- DOI: https://doi.org/10.1093/nar/gkaa541
- Primary Citation of Related Structures:
6S1C, 6S2E, 6S2F - PubMed Abstract:
The eukaryotic replisome must faithfully replicate DNA and cope with replication fork blocks and stalling, while simultaneously promoting sister chromatid cohesion. Ctf18-RFC is an alternative PCNA loader that links all these processes together by an unknown mechanism. Here, we use integrative structural biology combined with yeast genetics and biochemistry to highlight the specific functions that Ctf18-RFC plays within the leading strand machinery via an interaction with the catalytic domain of DNA Pol ϵ. We show that a large and unusually flexible interface enables this interaction to occur constitutively throughout the cell cycle and regardless of whether forks are replicating or stalled. We reveal that, by being anchored to the leading strand polymerase, Ctf18-RFC can rapidly signal fork stalling to activate the S phase checkpoint. Moreover, we demonstrate that, independently of checkpoint signaling or chromosome cohesion, Ctf18-RFC functions in parallel to Chl1 and Mrc1 to protect replication forks and cell viability.
- University of Warwick, Warwick Medical School, Coventry, UK.
Organizational Affiliation: