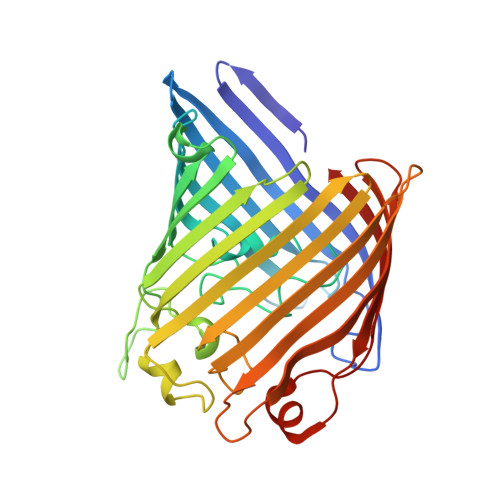
OmpK36-mediated Carbapenem resistance attenuates ST258 Klebsiella pneumoniae in vivo.
Wong, J.L.C., Romano, M., Kerry, L.E., Kwong, H.S., Low, W.W., Brett, S.J., Clements, A., Beis, K., Frankel, G.(2019) Nat Commun 10: 3957-3957
- PubMed: 31477712
- DOI: https://doi.org/10.1038/s41467-019-11756-y
- Primary Citation of Related Structures:
6RCK, 6RCP, 6RD3 - PubMed Abstract:
Carbapenem-resistance in Klebsiella pneumoniae (KP) sequence type ST258 is mediated by carbapenemases (e.g. KPC-2) and loss or modification of the major non-selective porins OmpK35 and OmpK36. However, the mechanism underpinning OmpK36-mediated resistance and consequences of these changes on pathogenicity remain unknown. By solving the crystal structure of a clinical ST258 OmpK36 variant we provide direct structural evidence of pore constriction, mediated by a di-amino acid (Gly115-Asp116) insertion into loop 3, restricting diffusion of both nutrients (e.g. lactose) and Carbapenems. In the presence of KPC-2 this results in a 16-fold increase in MIC to Meropenem. Additionally, the Gly-Asp insertion impairs bacterial growth in lactose-containing medium and confers a significant in vivo fitness cost in a murine model of ventilator-associated pneumonia. Our data suggests that the continuous selective pressure imposed by widespread Carbapenem utilisation in hospital settings drives the expansion of KP expressing Gly-Asp insertion mutants, despite an associated fitness cost.
- Centre for Molecular Bacteriology and Infection, Department of Life Sciences, Imperial College London, London, UK.
Organizational Affiliation: