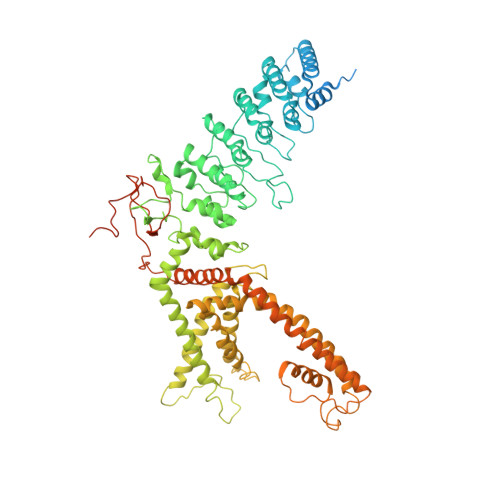
Structural basis of temperature sensation by the TRP channel TRPV3.
Singh, A.K., McGoldrick, L.L., Demirkhanyan, L., Leslie, M., Zakharian, E., Sobolevsky, A.I.(2019) Nat Struct Mol Biol 26: 994-998
- PubMed: 31636415
- DOI: https://doi.org/10.1038/s41594-019-0318-7
- Primary Citation of Related Structures:
6PVL, 6PVM, 6PVN, 6PVO, 6PVP, 6PVQ - PubMed Abstract:
We present structures of mouse TRPV3 in temperature-dependent open, closed and intermediate states that suggest two-step activation of TRPV3 by heat. During the strongly temperature-dependent first step, sensitization, the channel pore remains closed while S6 helices undergo α-to-π transitions. During the weakly temperature-dependent second step, channel opening, tight association of the S1-S4 and pore domains is stabilized by changes in the carboxy-terminal and linker domains.
- Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY, USA.
Organizational Affiliation: