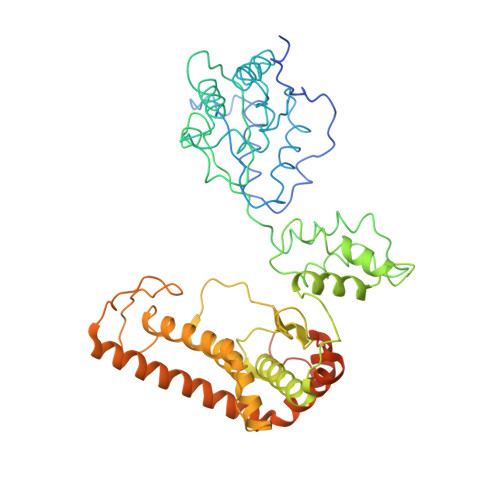
Unique Structural Features of the Mitochondrial AAA+ Protease AFG3L2 Reveal the Molecular Basis for Activity in Health and Disease.
Puchades, C., Ding, B., Song, A., Wiseman, R.L., Lander, G.C., Glynn, S.E.(2019) Mol Cell 75: 1073
- PubMed: 31327635
- DOI: https://doi.org/10.1016/j.molcel.2019.06.016
- Primary Citation of Related Structures:
6NYY - PubMed Abstract:
Mitochondrial AAA+ quality-control proteases regulate diverse aspects of mitochondrial biology through specialized protein degradation, but the underlying mechanisms of these enzymes remain poorly defined. The mitochondrial AAA+ protease AFG3L2 is of particular interest, as genetic mutations localized throughout AFG3L2 are linked to diverse neurodegenerative disorders. However, a lack of structural data has limited our understanding of how mutations impact enzymatic function. Here, we used cryoelectron microscopy (cryo-EM) to determine a substrate-bound structure of the catalytic core of human AFG3L2. This structure identifies multiple specialized structural features that integrate with conserved motifs required for ATP-dependent translocation to unfold and degrade targeted proteins. Many disease-relevant mutations localize to these unique structural features of AFG3L2 and distinctly influence its activity and stability. Our results provide a molecular basis for neurological phenotypes associated with different AFG3L2 mutations and establish a structural framework to understand how different members of the AAA+ superfamily achieve specialized biological functions.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA; Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037, USA; Skaggs Graduate School of Chemical and Biological Sciences, The Scripps Research Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: