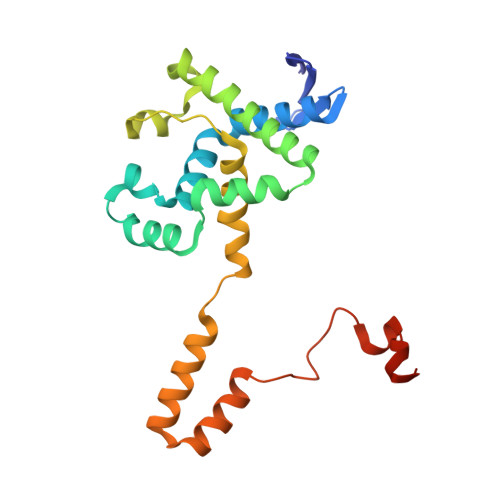
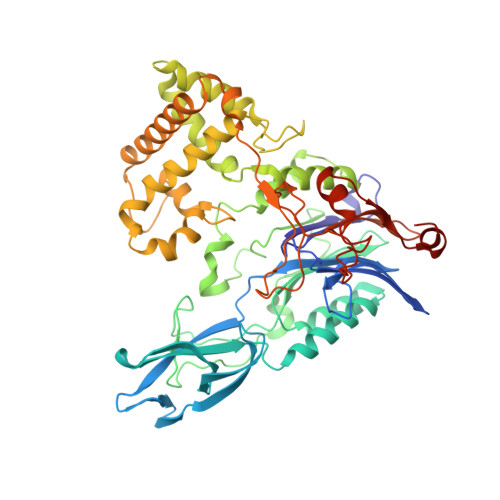
Crystal structures and protein engineering of three different penicillin G acylases from Gram-positive bacteria with different thermostability.
Mayer, J., Pippel, J., Gunther, G., Muller, C., Lauermann, A., Knuuti, T., Blankenfeldt, W., Jahn, D., Biedendieck, R.(2019) Appl Microbiol Biotechnol 103: 7537-7552
- PubMed: 31227867
- DOI: https://doi.org/10.1007/s00253-019-09977-8
- Primary Citation of Related Structures:
6NVW, 6NVX, 6NVY - PubMed Abstract:
Penicillin G acylase (PGA) catalyzes the hydrolysis of penicillin G to 6-aminopenicillanic acid and phenylacetic acid, which provides the precursor for most semisynthetic penicillins. Most applications rely on PGAs from Gram-negative bacteria. Here we describe the first three crystal structures for PGAs from Gram-positive Bacilli and their utilization in protein engineering experiments for the manipulation of their thermostability. PGAs from Bacillus megaterium (BmPGA, T m = 56.0 °C), Bacillus thermotolerans (BtPGA, T m = 64.5 °C), and Bacillus sp. FJAT-27231 (FJAT-PGA, T m = 74.3 °C) were recombinantly produced with B. megaterium, secreted, purified to apparent heterogeneity, and crystallized. Structures with resolutions of 2.20 Å (BmPGA), 2.27 Å (BtPGA), and 1.36 Å (FJAT-PGA) were obtained. They revealed high overall similarity, reflecting the high identity of up to approx. 75%. Notably, the active center displays a deletion of more than ten residues with respect to PGAs from Gram-negatives. This enlarges the substrate binding site and may indicate a different substrate spectrum. Based on the structures, ten single-chain FJAT-PGAs carrying artificial linkers were produced. However, in all cases, complete linker cleavage was observed. While thermostability remained in the wild-type range, the enzymatic activity dropped between 30 and 60%. Furthermore, four hybrid PGAs carrying subunits from two different enzymes were successfully produced. Their thermostabilities mostly lay between the values of the two mother enzymes. For one PGA increased, enzyme activity was observed. Overall, the three novel PGA structures combined with initial protein engineering experiments provide the basis for establishment of new PGA-based biotechnological processes.
- Institute of Microbiology and Braunschweig Integrated Centre of Systems Biology (BRICS), Technische Universität Braunschweig, Rebenring 56, 38106, Braunschweig, Germany.
Organizational Affiliation: