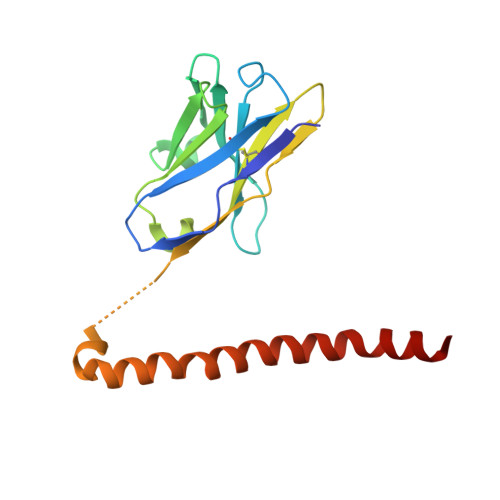
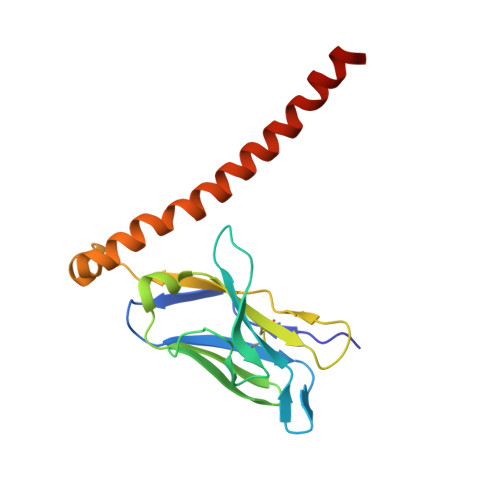
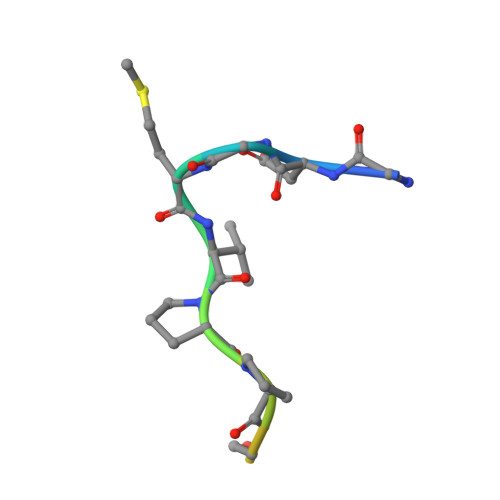
Site-specific epitope insertion into recombinant proteins using the MAP tag system.
Wakasa, A., Kaneko, M.K., Kato, Y., Takagi, J., Arimori, T.(2020) J Biochem 168: 375-384
- PubMed: 32386302
- DOI: https://doi.org/10.1093/jb/mvaa054
- Primary Citation of Related Structures:
6LZ4 - PubMed Abstract:
The MAP tag system comprises a 14-residue peptide derived from mouse podoplanin and its high-affinity monoclonal antibody PMab-1. We determined the crystal structure of PMab-1 complexed with the MAP tag peptide and found that the recognition required only the N-terminal 8 residues of MAP tag sequence, enabling the shortening of the tag length without losing the affinity for PMab-1. Furthermore, the structure illustrated that the MAP tag adopts a U-shaped conformation when bound by PMab-1, suggesting that loop-inserted MAP tag would assume conformation compatible with the PMab-1 binding. We inserted the 8-residue MAP tag into multiple loop regions in various proteins including fibronectin type III domain and G-protein-coupled receptors and tested if they maintain PMab-1 reactivity. Despite the conformational restraints forced by the insertion position, all MAP-inserted mutants were expressed well in mammalian cells at levels comparable to the non-tagged proteins. Furthermore, the binding by PMab-1 was fully maintained even for the mutant where MAP tag was inserted at a structurally restricted β-hairpin, indicating that the MAP tag system has unique feature that allows placement in the middle of protein domain at desired locations. Our results indicate the versatile utility of the MAP tag system in 'site-specific epitope insertion' application.
- Laboratory of Protein Synthesis and Expression, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Organizational Affiliation: