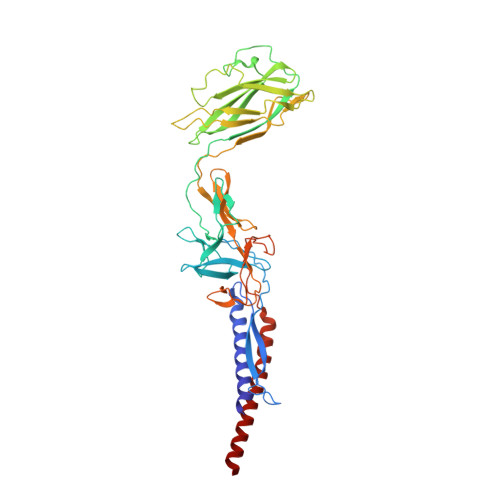
Structure ofSalmonellaFlagellar Hook Reveals Intermolecular Domain Interactions for the Universal Joint Function.
Horvath, P., Kato, T., Miyata, T., Namba, K.(2019) Biomolecules 9
- PubMed: 31505847
- DOI: https://doi.org/10.3390/biom9090462
- Primary Citation of Related Structures:
6KFK - PubMed Abstract:
The bacterial flagellum is a motility organelle consisting of a rotary motor and a long helical filament as a propeller. The flagellar hook is a flexible universal joint that transmits motor torque to the filament in its various orientations that change dynamically between swimming and tumbling of the cell upon switching the motor rotation for chemotaxis. Although the structures of the hook and hook protein FlgE from different bacterial species have been studied, the structure of Salmonella hook, which has been studied most over the years, has not been solved at a high enough resolution to allow building an atomic model of entire FlgE for understanding the mechanisms of self-assembly, stability and the universal joint function. Here we report the structure of Salmonella polyhook at 4.1 Å resolution by electron cryomicroscopy and helical image analysis. The density map clearly revealed folding of the entire FlgE chain forming the three domains D0, D1 and D2 and allowed us to build an atomic model. The model includes domain Dc with a long β-hairpin structure that connects domains D0 and D1 and contributes to the structural stability of the hook while allowing the flexible bending of the hook as a molecular universal joint.
- Graduate School of Frontier Biosciences, Osaka University, 1-3 Yamadaoka, Suita, Osaka 565-0871, Japan.
Organizational Affiliation: