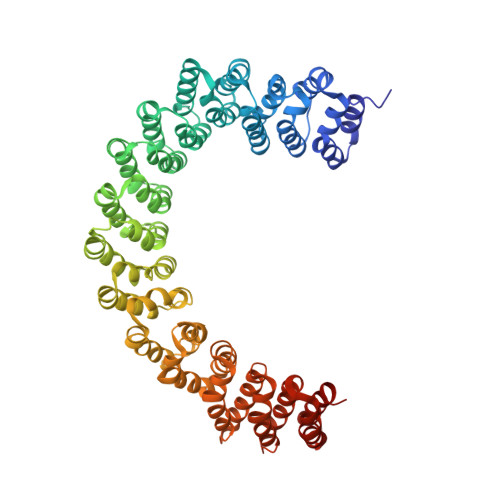Selective Inhibition of STRN3-Containing PP2A Phosphatase Restores Hippo Tumor-Suppressor Activity in Gastric Cancer.
Tang, Y., Fang, G., Guo, F., Zhang, H., Chen, X., An, L., Chen, M., Zhou, L., Wang, W., Ye, T., Zhou, L., Nie, P., Yu, H., Lin, M., Zhao, Y., Lin, X., Yuan, Z., Jiao, S., Zhou, Z.(2020) Cancer Cell 38: 115-128.e9
- PubMed: 32589942
- DOI: https://doi.org/10.1016/j.ccell.2020.05.019
- Primary Citation of Related Structures:
6IUR - PubMed Abstract:
Loss of Hippo tumor-suppressor activity and hyperactivation of YAP are commonly observed in cancers. Inactivating mutations of Hippo kinases MST1/2 are uncommon, and it remains unclear how their activity is turned off during tumorigenesis. We identified STRN3 as an essential regulatory subunit of protein phosphatase 2A (PP2A) that recruits MST1/2 and promotes its dephosphorylation, which results in YAP activation. We also identified STRN3 upregulation in gastric cancer correlated with YAP activation and poor prognosis. Based on this mechanistic understanding and aided by structure-guided medicinal chemistry, we developed a highly selective peptide inhibitor, STRN3-derived Hippo-activating peptide, or SHAP, which disrupts the STRN3-PP2Aa interaction and reactivates the Hippo tumor suppressor, inhibits YAP activation, and has antitumor effects in vivo.
- State Key Laboratory of Cell Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Shanghai 200031, China; Tongji University Cancer Center, Postdoctoral Station of Clinical Medicine, Department of Medical Ultrasound, Ultrasound Research and Education Institute, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai 200072, China.
Organizational Affiliation: