Direct Binding of the Flexible C-Terminal Segment of Periaxin to beta 4 Integrin Suggests a Molecular Basis for CMT4F.
Raasakka, A., Linxweiler, H., Brophy, P.J., Sherman, D.L., Kursula, P.(2019) Front Mol Neurosci 12: 84-84
- PubMed: 31024253
- DOI: https://doi.org/10.3389/fnmol.2019.00084
- Primary Citation of Related Structures:
6HYF - PubMed Abstract:
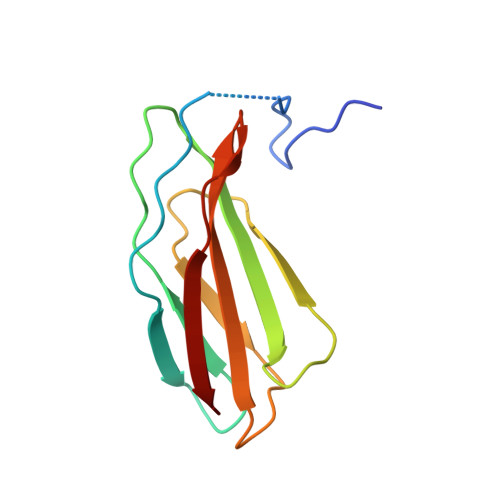
The process of myelination in the nervous system requires a coordinated formation of both transient and stable supramolecular complexes. Myelin-specific proteins play key roles in these assemblies, which may link membranes to each other or connect the myelinating cell cytoskeleton to the extracellular matrix. The myelin protein periaxin is known to play an important role in linking the Schwann cell cytoskeleton to the basal lamina through membrane receptors, such as the dystroglycan complex. Mutations that truncate periaxin from the C terminus cause demyelinating peripheral neuropathy, Charcot-Marie-Tooth (CMT) disease type 4F, indicating a function for the periaxin C-terminal region in myelination. We identified the cytoplasmic domain of β4 integrin as a specific high-affinity binding partner for periaxin. The C-terminal region of periaxin remains unfolded and flexible when bound to the third fibronectin type III domain of β4 integrin. Our data suggest that periaxin is able to link the Schwann cell cytoplasm to the basal lamina through a two-pronged interaction via different membrane protein complexes, which bind close to the N and C terminus of this elongated, flexible molecule.
- Department of Biomedicine, University of Bergen, Bergen, Norway.
Organizational Affiliation: