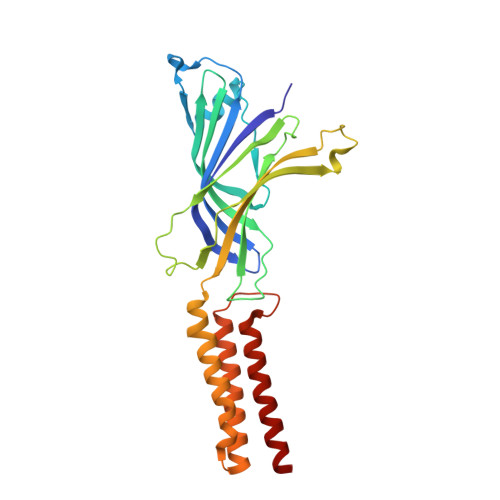
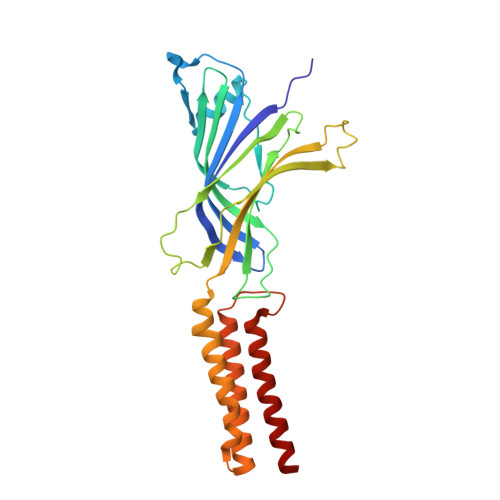
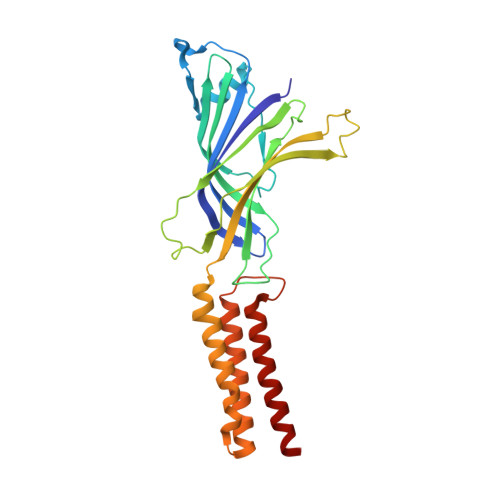
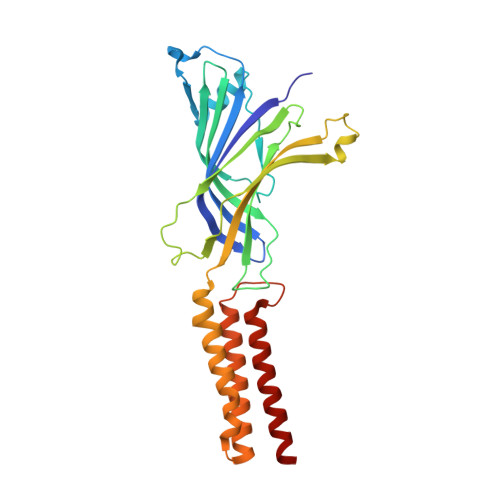
A lipid site shapes the agonist response of a pentameric ligand-gated ion channel.
Henault, C.M., Govaerts, C., Spurny, R., Brams, M., Estrada-Mondragon, A., Lynch, J., Bertrand, D., Pardon, E., Evans, G.L., Woods, K., Elberson, B.W., Cuello, L.G., Brannigan, G., Nury, H., Steyaert, J., Baenziger, J.E., Ulens, C.(2019) Nat Chem Biol 15: 1156-1164
- PubMed: 31591563
- DOI: https://doi.org/10.1038/s41589-019-0369-4
- Primary Citation of Related Structures:
6HJX, 6HJY, 6HK0 - PubMed Abstract:
Phospholipids are key components of cellular membranes and are emerging as important functional regulators of different membrane proteins, including pentameric ligand-gated ion channels (pLGICs). Here, we take advantage of the prokaryote channel ELIC (Erwinia ligand-gated ion channel) as a model to understand the determinants of phospholipid interactions in this family of receptors. A high-resolution structure of ELIC in a lipid-bound state reveals a phospholipid site at the lower half of pore-forming transmembrane helices M1 and M4 and at a nearby site for neurosteroids, cholesterol or general anesthetics. This site is shaped by an M4-helix kink and a Trp-Arg-Pro triad that is highly conserved in eukaryote GABA A/C and glycine receptors. A combined approach reveals that M4 is intrinsically flexible and that M4 deletions or disruptions of the lipid-binding site accelerate desensitization in ELIC, suggesting that lipid interactions shape the agonist response. Our data offer a structural context for understanding lipid modulation in pLGICs.
- Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Ontario, Canada.
Organizational Affiliation: