Self-protection of the catalytic iron center of a methanogenic [Fe]-hydrogenase via a dynamic dimer-to-hexamer transformation
Wagner, T., Huang, G., Ermler, U., Shima, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 5,10-methenyltetrahydromethanopterin hydrogenase | 344 | Methanothermobacter marburgensis str. Marburg | Mutation(s): 0 EC: 1.12.98.2 | 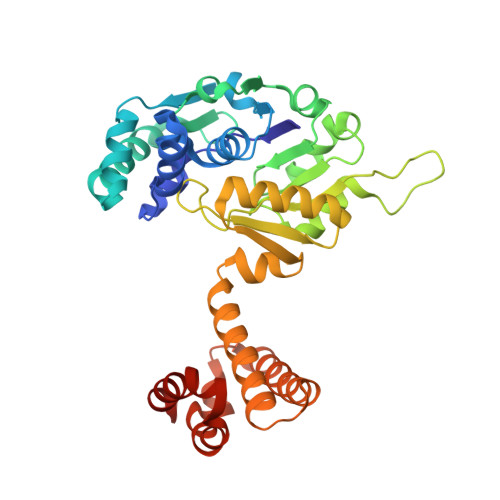 | |
UniProt | |||||
Find proteins for P32440 (Methanothermobacter marburgensis (strain ATCC BAA-927 / DSM 2133 / JCM 14651 / NBRC 100331 / OCM 82 / Marburg)) Explore P32440 Go to UniProtKB: P32440 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P32440 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FE9 Query on FE9 | F [auth A] | iron-guanylyl pyridinol cofactor C21 H23 Fe N6 O13 P S AEHOAZNVUAGELD-VPXBKTNXSA-K |  | ||
| MES Query on MES | B [auth A] | 2-(N-MORPHOLINO)-ETHANESULFONIC ACID C6 H13 N O4 S SXGZJKUKBWWHRA-UHFFFAOYSA-N |  | ||
| SO4 Query on SO4 | C [auth A], D [auth A], E [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | G [auth A], H [auth A], I [auth A], J [auth A], K [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 144.06 | α = 90 |
| b = 144.06 | β = 90 |
| c = 95.06 | γ = 120 |
| Software Name | Purpose |
|---|---|
| BUSTER | refinement |
| XDS | data reduction |
| SCALA | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Max Planck Society | Germany | -- |
| Germany | Deutsche Forschungsgemeinschaft Priority Program Iron Sulfur for Life (SH87/1-1) | |
| China | China Scholarship Council |