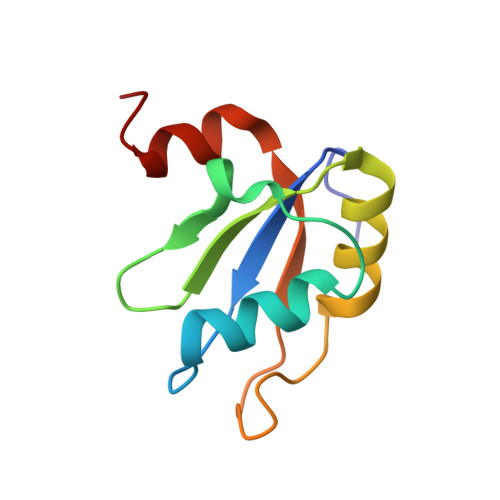
Crystal structure of human Acinus RNA recognition motif domain.
Fernandes, H., Czapinska, H., Grudziaz, K., Bujnicki, J.M., Nowacka, M.(2018) PeerJ 6: e5163-e5163
- PubMed: 30042883
- DOI: https://doi.org/10.7717/peerj.5163
- Primary Citation of Related Structures:
6G6S - PubMed Abstract:
Acinus is an abundant nuclear protein involved in apoptosis and splicing. It has been implicated in inducing apoptotic chromatin condensation and DNA fragmentation during programmed cell death. Acinus undergoes activation by proteolytic cleavage that produces a truncated p17 form that comprises only the RNA recognition motif (RRM) domain. We have determined the crystal structure of the human Acinus RRM domain (AcRRM) at 1.65 Å resolution. It shows a classical four-stranded antiparallel β-sheet fold with two flanking α-helices and an additional, non-classical α-helix at the C-terminus, which harbors the caspase-3 target sequence that is cleaved during Acinus activation. In the structure, the C-terminal α-helix partially occludes the potential ligand binding surface of the β-sheet and hypothetically shields it from non-sequence specific interactions with RNA. Based on the comparison with other RRM-RNA complex structures, it is likely that the C-terminal α-helix changes its conformation with respect to the RRM core in order to enable RNA binding by Acinus.
- International Institute of Molecular and Cell Biology in Warsaw, Warsaw, Poland.
Organizational Affiliation: