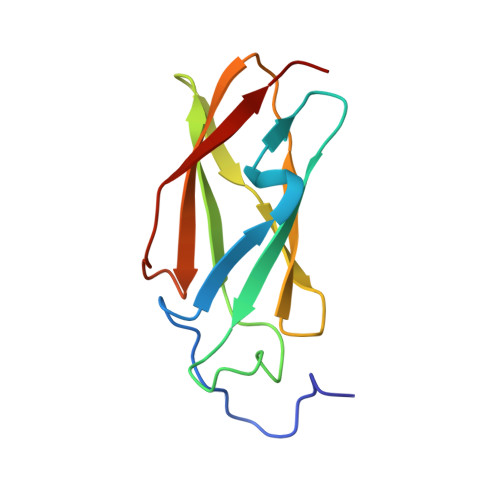
Mapping energy landscapes of a growing filamin domain reveals an intermediate associated with proline isomerization during biosynthesis
Waudby, C.A., Wlodarski, T., Karyadi, M.-E., Cassaignau, A.M.E., Chan, S.H.S., Wentink, A.S., Schmidt-Engler, J.M., Camilloni, C., Vendruscolo, M., Cabrita, L.D., Christodoulou, J.To be published.