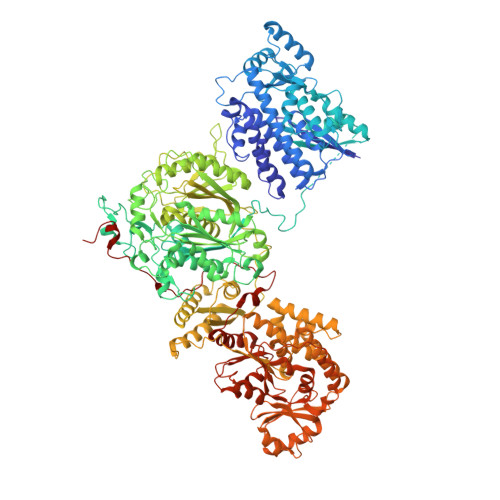
The structural organization of substrate loading in iterative polyketide synthases.
Herbst, D.A., Huitt-Roehl, C.R., Jakob, R.P., Kravetz, J.M., Storm, P.A., Alley, J.R., Townsend, C.A., Maier, T.(2018) Nat Chem Biol 14: 474-479
- PubMed: 29610486
- DOI: https://doi.org/10.1038/s41589-018-0026-3
- Primary Citation of Related Structures:
6FIJ, 6FIK - PubMed Abstract:
Polyketide synthases (PKSs) are microbial multienzymes for the biosynthesis of biologically potent secondary metabolites. Polyketide production is initiated by the loading of a starter unit onto an integral acyl carrier protein (ACP) and its subsequent transfer to the ketosynthase (KS). Initial substrate loading is achieved either by multidomain loading modules or by the integration of designated loading domains, such as starter unit acyltransferases (SAT), whose structural integration into PKS remains unresolved. A crystal structure of the loading/condensing region of the nonreducing PKS CTB1 demonstrates the ordered insertion of a pseudodimeric SAT into the condensing region, which is aided by the SAT-KS linker. Cryo-electron microscopy of the post-loading state trapped by mechanism-based crosslinking of ACP to KS reveals asymmetry across the CTB1 loading/-condensing region, in accord with preferential 1:2 binding stoichiometry. These results are critical for re-engineering the loading step in polyketide biosynthesis and support functional relevance of asymmetric conformations of PKSs.
- Department of Biozentrum, University of Basel, Basel, Switzerland.
Organizational Affiliation: