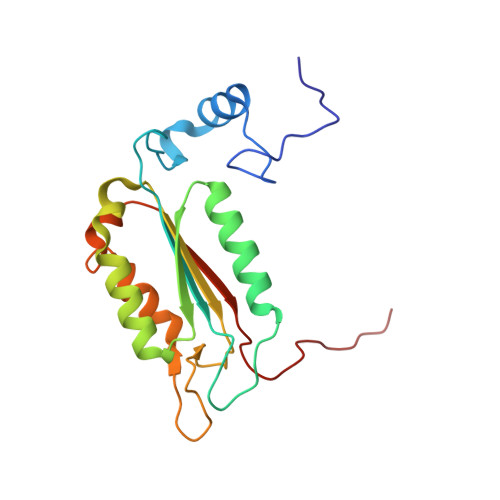
Rational Drug Design of Topically Administered Caspase 1 Inhibitors for the Treatment of Inflammatory Acne.
Fournier, J.F., Clary, L., Chambon, S., Dumais, L., Harris, C.S., Millois, C., Pierre, R., Talano, S., Thoreau, E., Aubert, J., Aurelly, M., Bouix-Peter, C., Brethon, A., Chantalat, L., Christin, O., Comino, C., El-Bazbouz, G., Ghilini, A.L., Isabet, T., Lardy, C., Luzy, A.P., Mathieu, C., Mebrouk, K., Orfila, D., Pascau, J., Reverse, K., Roche, D., Rodeschini, V., Hennequin, L.F.(2018) J Med Chem 61: 4030-4051
- PubMed: 29648825
- DOI: https://doi.org/10.1021/acs.jmedchem.8b00067
- Primary Citation of Related Structures:
6F6R - PubMed Abstract:
The use of an interleukin β antibody is currently being investigated in the clinic for the treatment of acne, a dermatological disorder affecting 650M persons globally. Inhibiting the protease responsible for the cleavage of inactive pro-IL1β into active IL-1β, caspase-1, could be an alternative small molecule approach. This report describes the discovery of uracil 20, a potent (38 nM in THP1 cells assay) caspase-1 inhibitor for the topical treatment of inflammatory acne. The uracil series was designed according to a published caspase-1 pharmacophore model involving a reactive warhead in P1 for covalent reversible inhibition and an aryl moiety in P4 for selectivity against the apoptotic caspases. Reversibility was assessed in an enzymatic dilution assay or by using different substrate concentrations. In addition to classical structure-activity-relationship exploration, topical administration challenges such as phototoxicity, organic and aqueous solubility, chemical stability in solution, and skin metabolic stability are discussed and successfully resolved.
- Nestlé Skin Health R&D , 2400 Route des Colles, BP 87 , 06902 Sophia-Antipolis Cedex, France.
Organizational Affiliation: