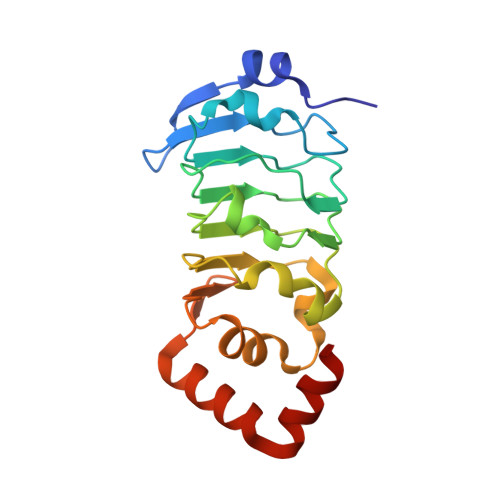
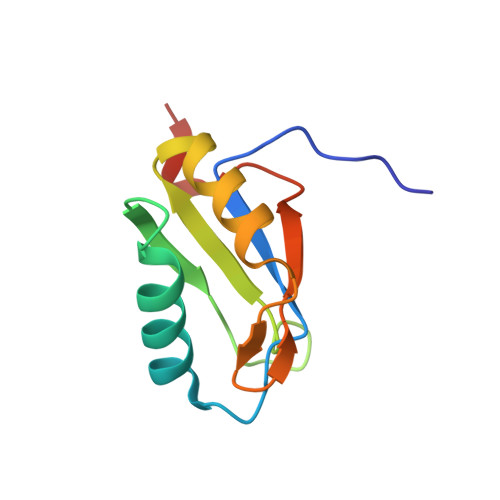
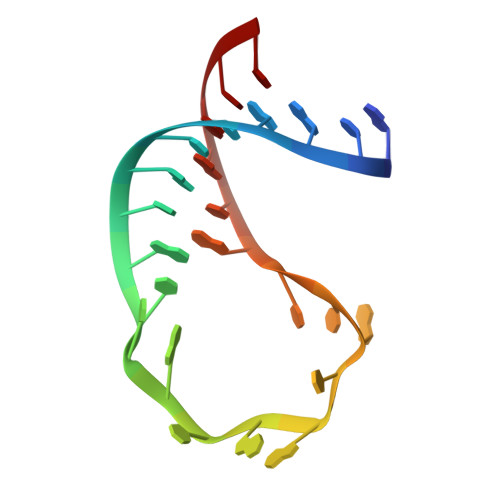
Molecular principles underlying dual RNA specificity in the Drosophila SNF protein.
Weber, G., DeKoster, G.T., Holton, N., Hall, K.B., Wahl, M.C.(2018) Nat Commun 9: 2220-2220
- PubMed: 29880797
- DOI: https://doi.org/10.1038/s41467-018-04561-6
- Primary Citation of Related Structures:
6F4G, 6F4H, 6F4I, 6F4J - PubMed Abstract:
The first RNA recognition motif of the Drosophila SNF protein is an example of an RNA binding protein with multi-specificity. It binds different RNA hairpin loops in spliceosomal U1 or U2 small nuclear RNAs, and only in the latter case requires the auxiliary U2A' protein. Here we investigate its functions by crystal structures of SNF alone and bound to U1 stem-loop II, U2A' or U2 stem-loop IV and U2A', SNF dynamics from NMR spectroscopy, and structure-guided mutagenesis in binding studies. We find that different loop-closing base pairs and a nucleotide exchange at the tips of the loops contribute to differential SNF affinity for the RNAs. U2A' immobilizes SNF and RNA residues to restore U2 stem-loop IV binding affinity, while U1 stem-loop II binding does not require such adjustments. Our findings show how U2A' can modulate RNA specificity of SNF without changing SNF conformation or relying on direct RNA contacts.
- Laboratory of Structural Biochemistry, Freie Universität Berlin, Takustraße 6, D-14195, Berlin, Germany. gweber@posteo.de.
Organizational Affiliation: