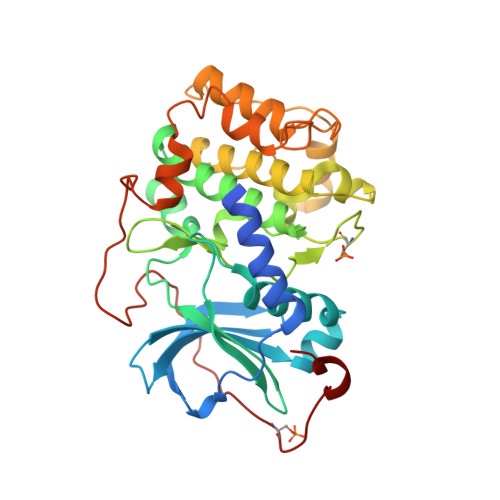
Identification of Selective Dual ROCK1 and ROCK2 Inhibitors Using Structure Based Drug Design.
Hobson, A.D., Judge, R.A., Aguirre, A.L., Brown, B.S., Cui, Y., Ding, P., Dominguez, E., DiGiammarino, E., Egan, D.A., Freiberg, G.M., Gopalakrishnan, S.M., Harris, C.M., Honore, M.P., Kage, K.L., Kapecki, N.J., Ling, C., Ma, J., Mack, H., Mamo, M., Maurus, S., McRae, B., Moore, N.S., Mueller, B.K., Mueller, R., Namovic, M.T., Patel, K., Pratt, S.D., Putman, C.B., Queeney, K.L., Sarris, K.K., Schaffter, L.M., Stoll, V.S., Vasudevan, A., Wang, L., Wang, L., Wirthl, W., Yach, K.(2018) J Med Chem 61: 11074-11100
- PubMed: 30384606
- DOI: https://doi.org/10.1021/acs.jmedchem.8b01098
- Primary Citation of Related Structures:
6E99, 6E9L, 6E9W, 6ED6 - PubMed Abstract:
A HTS campaign identified compound 1, an excellent hit-like molecule to initiate medicinal chemistry efforts to optimize a dual ROCK1 and ROCK2 inhibitor. Substitution (2-Cl, 2-NH 2 , 2-F, 3-F) of the pyridine hinge binding motif or replacement with pyrimidine afforded compounds with a clean CYP inhibition profile. Cocrystal structures of an early lead compound were obtained in PKA, ROCK1, and ROCK2. This provided critical structural information for medicinal chemistry to drive compound design. The structural data indicated the preferred configuration at the central benzylic carbon would be ( R), and application of this information to compound design resulted in compound 16. This compound was shown to be a potent and selective dual ROCK inhibitor in both enzyme and cell assays and efficacious in the retinal nerve fiber layer model after oral dosing. This tool compound has been made available through the AbbVie Compound Toolbox. Finally, the cocrystal structures also identified that aspartic acid residues 176 and 218 in ROCK2, which are glutamic acids in PKA, could be targeted as residues to drive both potency and kinome selectivity. Introduction of a piperidin-3-ylmethanamine group to the compound series resulted in compound 58, a potent and selective dual ROCK inhibitor with excellent predicted drug-like properties.
- AbbVie Bioresearch Center , 381 Plantation Street , Worcester , Massachusetts 01605 , United States.
Organizational Affiliation: