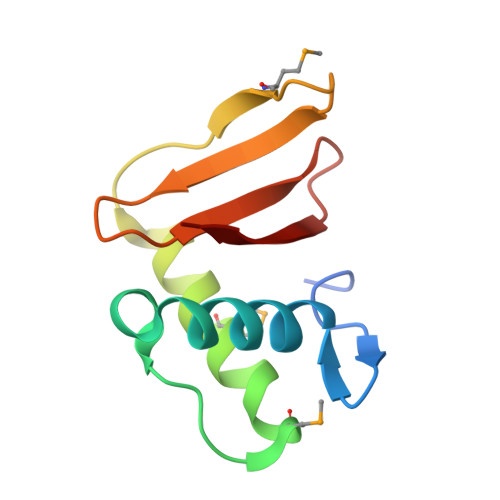
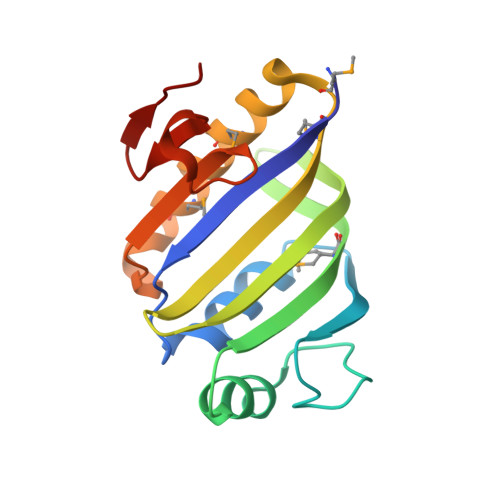
A comparative genomics approach identifies contact-dependent growth inhibition as a virulence determinant.
Allen, J.P., Ozer, E.A., Minasov, G., Shuvalova, L., Kiryukhina, O., Satchell, K.J.F., Hauser, A.R.(2020) Proc Natl Acad Sci U S A 117: 6811-6821
- PubMed: 32156726
- DOI: https://doi.org/10.1073/pnas.1919198117
- Primary Citation of Related Structures:
6D7Y - PubMed Abstract:
Emerging evidence suggests the Pseudomonas aeruginosa accessory genome is enriched with uncharacterized virulence genes. Identification and characterization of such genes may reveal novel pathogenic mechanisms used by particularly virulent isolates. Here, we utilized a mouse bacteremia model to quantify the virulence of 100 individual P. aeruginosa bloodstream isolates and performed whole-genome sequencing to identify accessory genomic elements correlated with increased bacterial virulence. From this work, we identified a specific contact-dependent growth inhibition (CDI) system enriched among highly virulent P. aeruginosa isolates. CDI systems contain a large exoprotein (CdiA) with a C-terminal toxin (CT) domain that can vary between different isolates within a species. Prior work has revealed that delivery of a CdiA-CT domain upon direct cell-to-cell contact can inhibit replication of a susceptible target bacterium. Aside from mediating interbacterial competition, we observed our virulence-associated CdiA-CT domain to promote toxicity against mammalian cells in culture and lethality during mouse bacteremia. Structural and functional studies revealed this CdiA-CT domain to have in vitro tRNase activity, and mutations that abrogated this tRNAse activity in vitro also attenuated virulence. Furthermore, CdiA contributed to virulence in mice even in the absence of contact-dependent signaling. Overall, our findings indicate that this P. aeruginosa CDI system functions as both an interbacterial inhibition system and a bacterial virulence factor against a mammalian host. These findings provide an impetus for continued studies into the complex role of CDI systems in P. aeruginosa pathogenesis.
- Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL 60611; jallen19@luc.edu.
Organizational Affiliation: