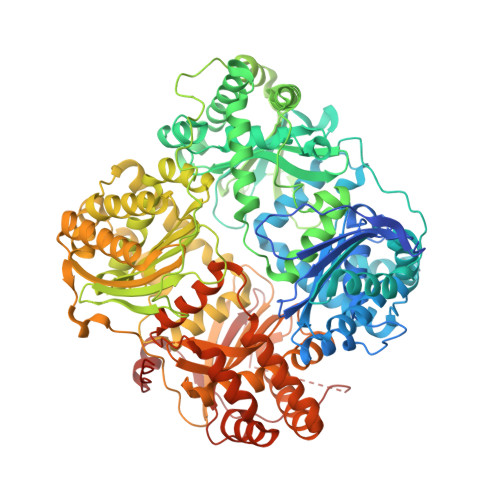
Substrate-selective inhibitors that reprogram the activity of insulin-degrading enzyme.
Maianti, J.P., Tan, G.A., Vetere, A., Welsh, A.J., Wagner, B.K., Seeliger, M.A., Liu, D.R.(2019) Nat Chem Biol 15: 565-574
- PubMed: 31086331
- DOI: https://doi.org/10.1038/s41589-019-0271-0
- Primary Citation of Related Structures:
6BYZ, 6EDS, 6MQ3 - PubMed Abstract:
Enzymes that act on multiple substrates are common in biology but pose unique challenges as therapeutic targets. The metalloprotease insulin-degrading enzyme (IDE) modulates blood glucose levels by cleaving insulin, a hormone that promotes glucose clearance. However, IDE also degrades glucagon, a hormone that elevates glucose levels and opposes the effect of insulin. IDE inhibitors to treat diabetes, therefore, should prevent IDE-mediated insulin degradation, but not glucagon degradation, in contrast with traditional modes of enzyme inhibition. Using a high-throughput screen for non-active-site ligands, we discovered potent and highly specific small-molecule inhibitors that alter IDE's substrate selectivity. X-ray co-crystal structures, including an IDE-ligand-glucagon ternary complex, revealed substrate-dependent interactions that enable these inhibitors to potently block insulin binding while allowing glucagon cleavage, even at saturating inhibitor concentrations. These findings suggest a path for developing IDE-targeting therapeutics, and offer a blueprint for modulating other enzymes in a substrate-selective manner to unlock their therapeutic potential.
- Merkin Institute of Transformative Technologies in Healthcare, Broad Institute of MIT and Harvard, Cambridge, MA, USA.
Organizational Affiliation: