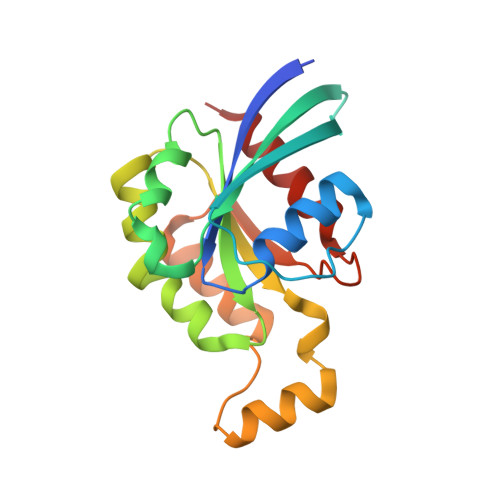
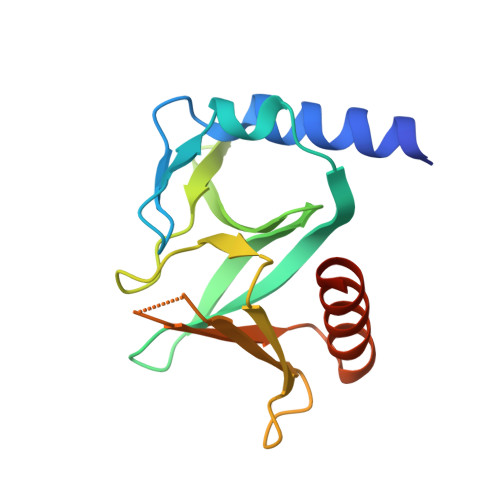
Crystal structures of the PH domains from Lbc family of RhoGEFs bound to activated RhoA GTPase.
Chen, Z., Gutowski, S., Sternweis, P.C.(2018) Data Brief 17: 356-362
- PubMed: 29876405
- DOI: https://doi.org/10.1016/j.dib.2018.01.024
- Primary Citation of Related Structures:
6BCA, 6BCB - PubMed Abstract:
The Pleckstrin homology (PH) domains from the Lbc family of Rho Guanine Nucleotide Exchange Factors (Lbc RhoGEFs) interact with activated Rho family GTPases. All 7 Lbc RhoGEFs associate directly with activated Rho GTPases via their PH domains. However, the binding affinities between the PH domains and the GTPases vary greatly. Here we present two crystal structures at resolutions of 1.4 Å and 2.0 Å of RhoA complexed with the PH domain from p114RhoGEF (PDB access code 6BCB) and AKAP-LbcRhoGEF (PDB access code 6BCA), respectively. These high resolution structures, together with the earlier structures of PDZRhoGEF-PH·RhoA and p190RhoGEF-PH·RhoA complexes, identify a highly conserved interface between the PH domains from Lbc-RhoGEFs and activated Rho GTPases. This manuscript is related to the manuscript titled "Direct Regulation of p190RhoGEF by Activated Rho and Rac GTPases" published in the Journal of Structural Biology.
- Department of Biophysics, The University of Texas Southwestern Medical Center, 6001 Forest Park Road, Dallas, TX 75390, United States.
Organizational Affiliation: